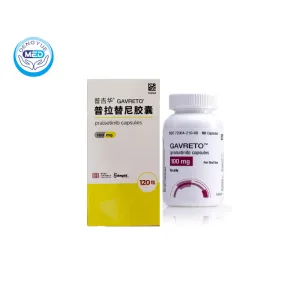
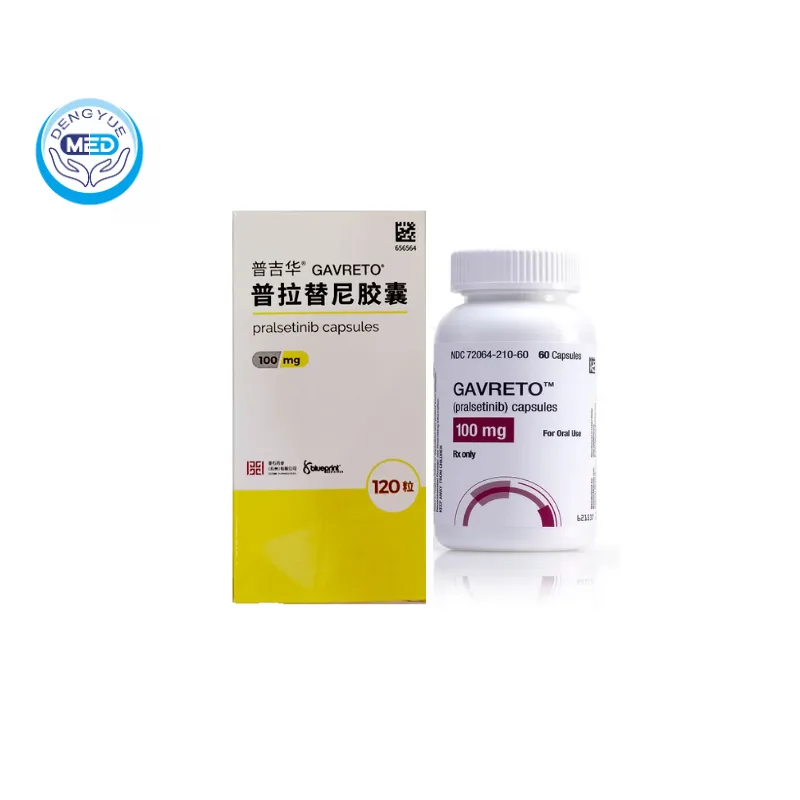
Gavreto (Pralsetinib) – NSCLC | HongKong DengYue Medicine
- Generic Name/Brand Name: Pralsetinib/Gavreto
- Indications: NSCLC (Lung Cancer)
- Dosage Form: Hard gelatin/HPMC capsules
- Specification: 100 mg (60/120 capsules)
Gavreto Application Scope
Gavreto is an oral, selective RET kinase inhibitor used to treat RET fusion–positive cancers.
Characteristics
-
Ingredients: Pralsetinib
-
Properties:
-
Oral kinase inhibitor targeting RET fusions and mutations
-
Capsules are light blue, opaque HPMC hard shells printed with “BLU‑667 100 mg”
-
-
Packaging Specification: Supplied as 100 mg capsules in bottles (e.g., 30-count) or blister packs
-
Storage: Store at room temperature; no special handling needed
-
Expiry Date: Use the expiry date printed on the packaging
-
Executive Standard: U.S. FDA-approved label; EMA and other agencies’ summaries align
-
Approval Number: FDA initial approval for NSCLC: September 4, 2020 (NDA 213721)
-
Date of Revision:
-
Latest U.S. label revision: July 2023 updates for CYP3A dose adjustments
-
Canadian revision May 30, 2024
-
-
Manufacturer:
-
Developed by Blueprint Medicines; marketed by Genentech (Roche) in the U.S.
-
Hoffmann‑La Roche in Canada, and Roche in other countries
-
Guidelines for the Use of Gavreto
-
Dosage and Administration:
-
400 mg orally once daily on an empty stomach (2 hours before and 1 hour after food)
-
Continue until disease progression or unacceptable toxicity
-
-
Adverse Reactions:
-
Common (≥ 25%):
-
fatigue, constipation, musculoskeletal pain, hypertension, peripheral edema
-
-
Lab abnormalities:
-
decreased blood counts, low calcium, sodium, phosphate; elevated liver enzymes
-
-
-
Contraindications: None specified in U.S. label
-
Precautions:
-
Interstitial lung disease (12% incidence; 3.3% Grade 3–4)
-
Monitor blood pressure (common hypertension)
-
Hepatotoxicity and hemorrhagic events require dose modifications or discontinuation
-
Gavreto Interactions
-
Drug Interactions:
-
Avoid strong/moderate CYP3A and P-gp inhibitors (reduce dose)
-
Avoid inducers (consider doubling the dose)
-
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.








Reviews
There are no reviews yet.