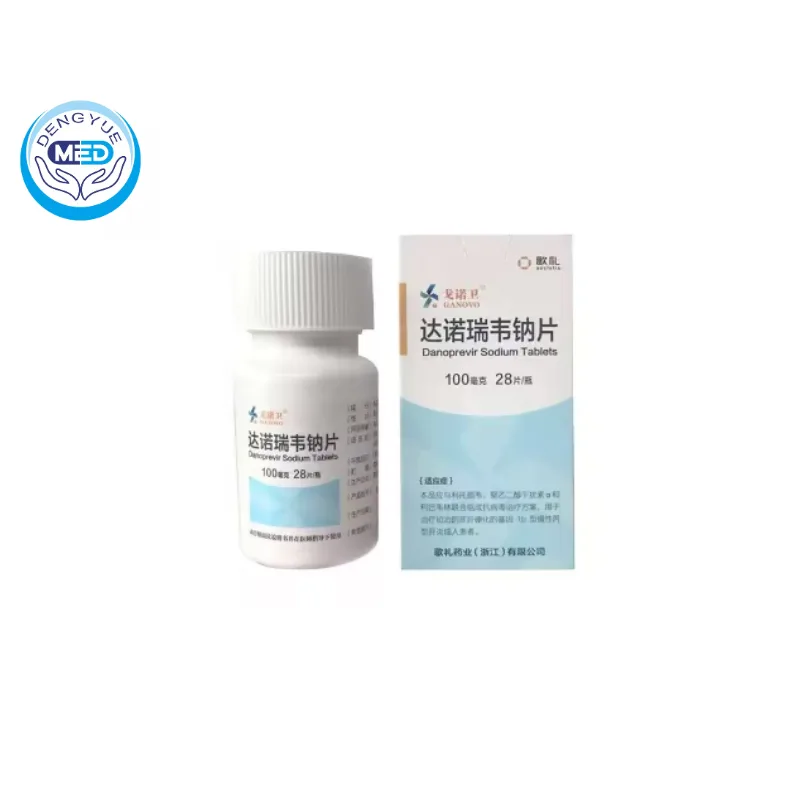
Ganovo (Danoprevir) – HCV | HongKong DengYue Medicine
- Generic Name/Brand Name: Danoprevir / Ganovo
- Indications: HCV
- Dosage Form: Capsule
- Specification: 100 mg
Ganovo Application Scope
Ganovo is an orally administered hepatitis C virus (HCV) NS3/4A protease inhibitor. It was the first domestically developed direct-acting antiviral (DAA) for HCV approved in China.

Characteristics
-
Ingredients: Danoprevir
-
Properties: Danoprevir is a potent inhibitor of the HCV NS3/4A protease, which is essential for viral replication.
-
Packaging Specification: Typically supplied in blister packs containing 100 mg film-coated tablets.
-
Storage: Store in a cool, dry place away from direct sunlight.
-
Expiry Date: Refer to the packaging for the specific expiration date.
-
Executive Standard: Manufactured in compliance with Good Manufacturing Practices (GMP)
-
Approval Number: Approved by the National Medical Products Administration (NMPA) of China
-
Date of Revision: Refer to the latest prescribing information leaflet for the date of revision.
-
Manufacturer: Ascletis Pharma Inc., China.
Guidelines for the Use of Ganovo
-
Dosage and Administration:
-
The recommended dosage is 100 mg taken orally twice daily
-
In combination with other antiviral agents such as ritonavir and peginterferon alfa-2a plus ribavirin
-
Depending on the specific treatment regimen
-
-
Adverse Reactions:
-
Common side effects may include fatigue, headache, nausea, and diarrhea.
-
Serious adverse reactions are rare but can include liver enzyme elevations.
-
-
Contraindications: Contraindicated in patients with known hypersensitivity to danoprevir or any component of the formulation.
-
Precautions:
-
Use with caution in patients with moderate to severe hepatic impairment.
-
Regular monitoring of liver function tests is recommended during treatment.
-
Ganovo Interactions
-
Drug Interactions:
-
Danoprevir is metabolized by the CYP3A4 enzyme
-
Co-administration with strong CYP3A4 inhibitors or inducers may affect drug levels
-
It is often administered with ritonavir to boost its plasma concentration
-
Careful consideration should be given when combining with other medications metabolized by CYP3A4
-
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.






Reviews
There are no reviews yet.