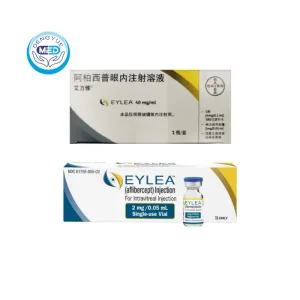
Eylea (aflibercept) – Diabetic Retinopathy | HongKong DengYue Medicine
- Generic Name/Brand Name: Aflibercept / Eylea®
- Indications:DR,DME
- Dosage Form: Intravitreal Injection
- Specification: 2 mg/0.05 mL solution (40 mg/mL) x single-dose prefilled syringe
Aflibercept Application Scope
Eylea (aflibercept) is indicated for the treatment of: neovascular (wet) age‑related macular degeneration (AMD), macular edema following retinal vein occlusion (RVO), diabetic macular edema (DME), diabetic retinopathy (DR), and retinopathy of prematurity (ROP) in preterm infants.
Aflibercept Characteristics
-
Ingredients:Active: aflibercept (recombinant fusion protein VEGFR‑1/VEGFR‑2 extracellular domains fused to human IgG1 Fc) at 40 mg/mL (2 mg/0.05 mL dose).
Excipients (vial/prefilled syringe formulations, 40 mg/mL): sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate heptahydrate, sodium chloride, sucrose, water for injections. -
Properties:Sterile, clear to pale yellow aqueous solution; preservative‑free; administered via intravitreal injection.
-
Packaging Specification:Single‑dose vial or single‑dose pre‑filled glass syringe: 2 mg (0.05 mL) of aflibercept at 40 mg/mL.
-
Storage:Store refrigerated at 2 °C to 8 °C; do not freeze; keep in original carton to protect from light. Unopened vial may be kept at ≤ 25 °C for up to 24 hours.
-
Expiry Date:Shelf life: 2 years from manufacture. Expiry is the last day of the stated month.
-
Executive Standard: EU marketing authorization number EU/1/12/797/001.
-
Approval Number:FDA: BLA 125387, first approved November 18, 2011 (US label updated October 2024).
-
Date of Revision:US prescribing information revised October 2024. EU product monograph revision April 14, 2025.
-
Manufacturer:Regeneron Pharmaceuticals, Inc. (US); Marketing Authorization Holder: Bayer AG, Leverkusen, Germany (EU).
Guidelines for the Use of Eylea
-
Dosage and Administration:
-
Recommended Dose: 2 mg (0.05 mL of 40 mg/mL solution) intravitreal injection once every 4 weeks (≈28 days) for initial induction (e.g., first 3 months for AMD, first 5 injections for DME/DR), followed by maintenance dosing every 8 weeks. Some patients may require continuing monthly dosing beyond initial period.
-
Administration: Administer via intravitreal injection using single‑dose vial or pre‑filled syringe under aseptic conditions by a qualified physician. Discard unused portion.
-
Missed Dose: If a dose is missed, administer as soon as possible and resume monthly dosing from the date of the most recently administered dose (aligns with “every 4‑week” guidance).
-
-
Adverse Reactions:
Common Adverse Reactions (≥5% and >3% higher than placebo):
Conjunctival hemorrhage, eye pain, cataract, vitreous detachment, floaters, ocular hypertension.(These ocular injection‑related events often meet incidence thresholds.)
Serious Adverse Reactions:
Hypersensitivity reactions, including bronchospasm, diffuse erythema, facial swelling, urticaria, chills, myalgias. Endophthalmitis and retinal detachment have been reported following injection.
-
Contraindications:Patients with a history of serious hypersensitivity to olezarsen or any of the excipients in TRYNGOLZA.
-
Precautions:
-
Hypersensitivity Reactions: Monitor for symptoms; discontinue use if reaction occurs.
-
Platelet Count Monitoring: Although systemic exposure is minimal, monitor as appropriate in patients with risk factors.
-
Pregnancy and Lactation: Advise patients to inform HCP if pregnant, planning pregnancy, or breastfeeding.
-
Aflibercep Interactions
- No known significant drug interactions described with intravitreal aflibercept.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.








Reviews
There are no reviews yet.