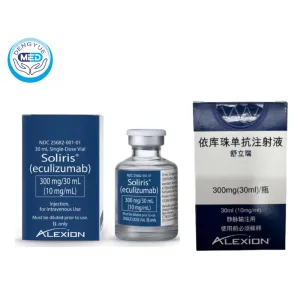
Eculizumab Injection | Paroxysmal Sleep Hemoglobinuria
- Generic Name/Brand Name: Eculizumab / Soliris
- Indications: PNH in Hematology, aHUS, gMG (anti-AChR antibody-positive), and NMOSD (anti-AQP4 antibody-positive
- Dosage Form: Injection
- Specification: 300 mg/30 mL (10 mg/mL) in a single-use vial
Eculizumab Injection Application Scope
Eculizumab Injection, marketed under the brand name Soliris, is a monoclonal antibody that inhibits the complement system, specifically targeting the C5 protein. It is primarily used to treat conditions involving uncontrolled complement activation.
- Paroxysmal Nocturnal Hemoglobinuria (PNH): A rare, life-threatening blood disorder characterized by the destruction of red blood cells. Eculizumab reduces hemolysis in these patients.
- Atypical Hemolytic Uremic Syndrome (aHUS): A condition leading to kidney failure due to abnormal blood clot formation. Eculizumab inhibits complement-mediated thrombotic microangiopathy in aHUS patients.
- Generalized Myasthenia Gravis (gMG): An autoimmune neuromuscular disorder causing weakness in skeletal muscles. Eculizumab is used in adults who are anti-acetylcholine receptor antibody-positive.
- Neuromyelitis Optica Spectrum Disorder (NMOSD): An inflammatory disorder of the central nervous system affecting the optic nerves and spinal cord. Eculizumab is indicated for patients who are anti-aquaporin-4 antibody-positive.
Eculizumab Injection Characteristics
- Ingredients: Active ingredient: Eculizumab. Inactive ingredients include polysorbate 80, sodium phosphate, and water for injection.
- Properties: Eculizumab is a humanized monoclonal IgG2/4κ antibody that binds to the complement protein C5, preventing its cleavage and subsequent formation of the membrane attack complex.
- Specification: Each vial contains 300 mg of eculizumab in 30 mL solution (10 mg/mL).
- Packaging Specification: Supplied in single-use vials; each carton contains one vial.
- Storage: Store vials in a refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze.
- Expiry Date: Refer to the expiration date printed on the vial and carton.
- Executive Standard: Manufactured in accordance with Good Manufacturing Practices (GMP) and approved by regulatory authorities such as the FDA and EMA.
- Approval Number: Refer to the specific regulatory approval number provided by the local health authority.
- Date of Revision: Refer to the latest revision date on the prescribing information leaflet.
- Manufacturer: Alexion Pharmaceuticals, Inc.
Guidelines for the Use of Eculizumab Injection
- Dosage and Administration:
- PNH and aHUS in Adults:
- Initial phase: 600 mg weekly for the first 4 weeks.
- Maintenance phase: 900 mg in the fifth week, followed by 900 mg every two weeks thereafter.
- gMG and NMOSD in Adults:
- Initial phase: 900 mg weekly for the first 4 weeks.
- Maintenance phase: 1,200 mg in the fifth week, followed by 1,200 mg every two weeks thereafter.
- Administer via intravenous infusion over 35 minutes. Patients should be monitored for at least one hour post-infusion for signs of infusion reactions.
- PNH and aHUS in Adults:
- Adverse Reactions:
- Common: Headache, nausea, pyrexia (fever), upper respiratory tract infections.
- Serious: Meningococcal infections, sepsis, and other serious infections.
Medication Limitations
- Contraindications:
- Patients with unresolved Neisseria meningitidis infection.
- Individuals who are not vaccinated against meningococcal infections unless the risks of delaying eculizumab injection therapy outweigh the risks of developing a meningococcal infection.
- Precautions:
- Vaccinate against meningococcal infections at least two weeks prior to initiating therapy.
- Monitor for early signs of meningococcal infections during treatment.
- Use caution in patients with systemic infections.
Eculizumab Injection Interactions
No formal drug interaction studies have been conducted. However, combining eculizumab with immunosuppressive therapies may increase the risk of infections. Consult healthcare providers for guidance on concomitant medications.
Note: If the drug has new packaging, the new packaging shall prevail. The above information is sourced from DengYueMed. It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.







Reviews
There are no reviews yet.