Cidofovir Injection | Anti CMV,AIDS | DengYueMed
- Generic Name/Brand Name: Cidofovir/Vistide
- Indications: Cytomegalovirus (CMV) retinitis in patients with AIDS.
- Dosage Form: Intravenous (IV) solution
- Specification: 75 mg/mL
Cidofovir Injection Application Scope
Cidofovir is an antiviral medication primarily used for the treatment of cytomegalovirus (CMV) retinitis in patients with acquired immunodeficiency syndrome (AIDS).
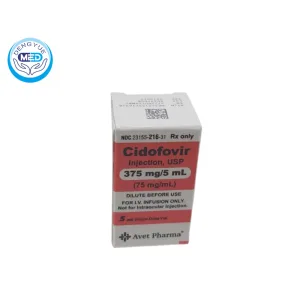
Cidofovir Injection Characteristics
- Ingredients: Cidofovir.
- Properties: It is an antiviral agent that inhibits viral DNA replication by selectively inhibiting viral DNA polymerases.
- Specification: Available as an intravenous solution.
- Packaging Specification: Specific packaging details should be referred to in the official prescribing information.
- Storage: Store at controlled room temperature, away from light.
- Expiry Date: Refer to the packaging for the specific expiry date.
- Executive Standard: Refer to the official prescribing information for detailed standards.
- Approval Number: Refer to the official prescribing information for approval details.
- Date of Revision: Refer to the official prescribing information for the revision date.
- Manufacturer: Gilead Sciences.
Guidelines For The Use Of Cidofovir Injection
Dosage and Administration:
- Induction Treatment: Administer 5 mg/kg body weight as an intravenous infusion over 1 hour, once weekly for two consecutive weeks.
- Maintenance Treatment: Starting two weeks after induction, administer 5 mg/kg body weight intravenously over 1 hour, once every two weeks.
- Administration Requirements: Prior to each dose, administer 1 liter of normal saline intravenously over 1 to 2 hours. If tolerated, a second liter may be given over 1 to 3 hours at the start or immediately after the cidofovir infusion. Concomitant administration of oral probenecid is recommended to reduce nephrotoxicity.
Adverse Reactions:
- Nephrotoxicity: Renal impairment is a significant risk; monitor renal function closely.
- Neutropenia: Decreased neutrophil counts may occur; regular blood counts are advised.
- Ophthalmic Issues: Potential for uveitis and decreased intraocular pressure.
- Other Effects: Nausea, vomiting, headache, and hair loss have been reported.
Contraindications:
- Hypersensitivity: Contraindicated in patients with known hypersensitivity to cidofovir or probenecid.
- Renal Impairment: Not recommended for patients with existing renal dysfunction.
- Concomitant Nephrotoxic Drugs: Avoid use with other nephrotoxic agents.
Precautions:
- Hydration and Probenecid: Ensure adequate hydration and administer probenecid as prescribed to mitigate nephrotoxic risks.
- Laboratory Monitoring: Regular renal function tests and complete blood counts are essential during treatment.
- Pregnancy Category: Category D; may cause harm to the fetus. Use only if the potential benefit justifies the potential risk to the fetus.
Cidofovir Injection Interactions
Drug Interactions:
- Nephrotoxic Agents: Concurrent use with other nephrotoxic drugs (e.g., amphotericin B, foscarnet, IV aminoglycosides, vancomycin, tacrolimus, NSAIDs) may increase the risk of renal toxicity.
- Probenecid: Cidofovir must be administered with probenecid, which inhibits renal tubular secretion, reducing nephrotoxicity.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from HongKong DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.
Contact Us







Reviews
There are no reviews yet.