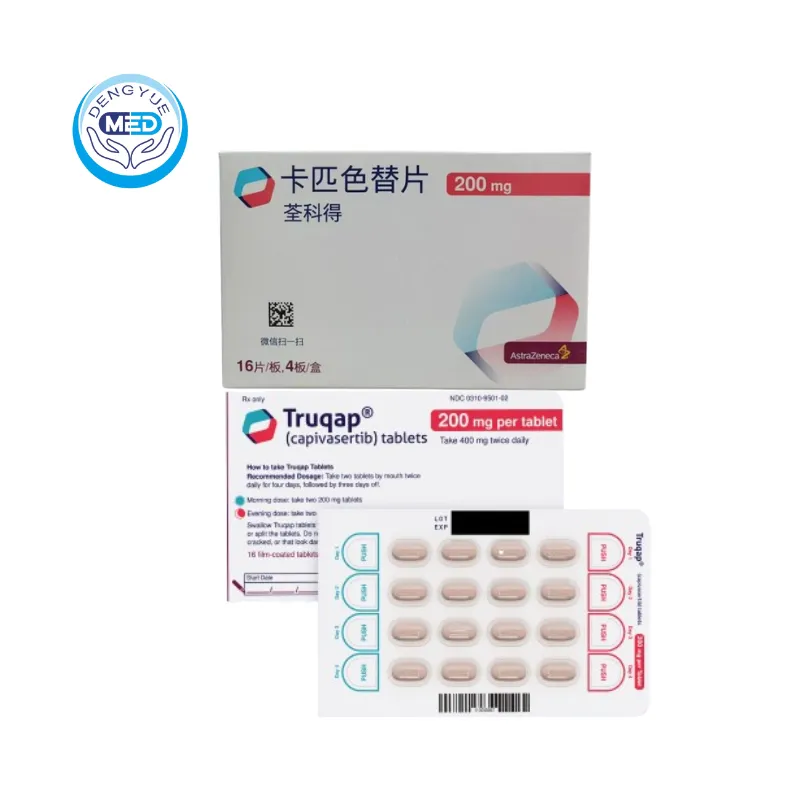
Capecitabine Tablets|Metastatic Breast Cancer
- Generic Name/Brand Name: Capecitabine Tablets/Xeloda
- Indications: Metastatic Breast Cancer
- Dosage Form: Tablets
- Specification: 500mg*30 tablets
Capecitabine Tablets Application Scope
Capecitabine Tablets are used for the treatment of metastatic breast cancer that is resistant to common therapies, and for the treatment of metastatic colorectal cancer in combination with Tazodi for the treatment of advanced breast cancer.
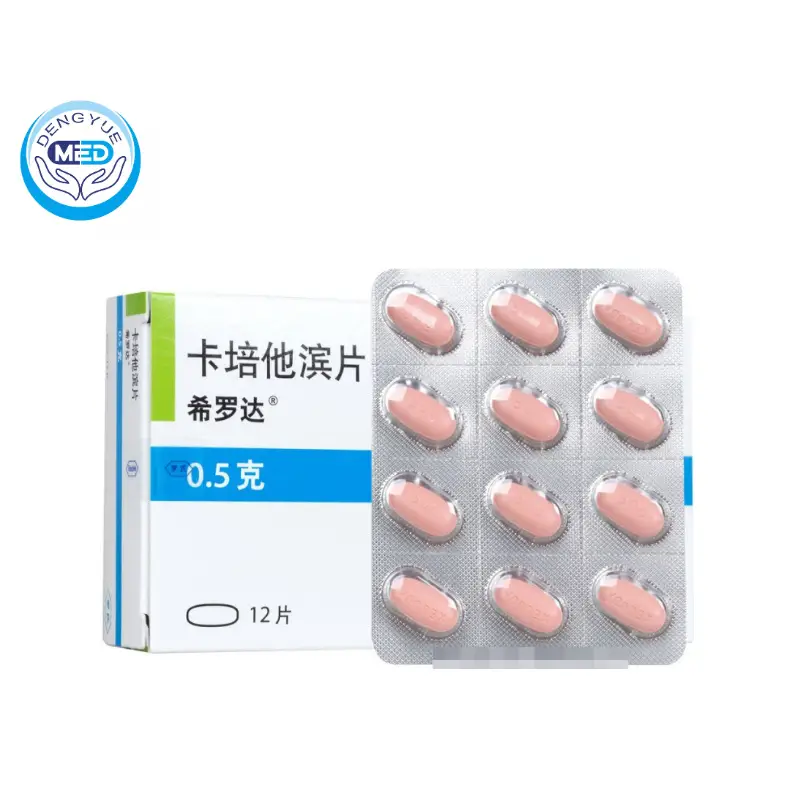
Features of Capecitabine Tablets
Ingredients: Capecitabine
Characteristics: Treatment of advanced breast cancer, metastatic breast cancer, metastatic colorectal cancer
Specification: 0.5g
Packing specification: 500mg*30 tablets
Storage: hermetically sealed
Effective period: 24 months
Execution Standard: YBH08582008
Approval code: State Drug License H20073024
Revision date According to the latest drug instruction.
Manufacturer: Roche Pharmaceuticals
Capecitabine Tablets Directions for Use
Dosage and administration: 2,500mg/m2 daily for two weeks with one week rest. The total daily dose should be swallowed with water in the morning and evening, half an hour after meals.
Adverse reactions: Gastrointestinal reactions such as diarrhea, nausea, vomiting, abdominal pain, and gastritis.
General adverse reactions: fatigue is frequent but severe is rare. Other common adverse reactions are mucositis, fever, weakness, and drowsiness but none are serious.
Guidelines For The Use Of Capecitabine Tablets
Contraindications: Shiroda is contraindicated in individuals with a history of allergy
Precautions: during the infusion process, the patient’s reaction should be closely observed, and cardiac patients should be used with caution.
Capecitabine Tablets Interactions
No clinically significant side effects have been observed when Shiroda is used in combination with a large number of drugs, such as antihistamines, NSAIDs, morphine, paracetamol, aspirin, antiemetics, and H2 receptor antagonists.
Note: If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYueMedicne, HongKong Drug Wholesale Distributor. It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.




Reviews
There are no reviews yet.