Bicalutamide Tablets | Advanced Prostate Cancer
- Generic Name/Brand Name: Bicalutamide/ZhaoHuiXian
- Indications: Luteinizing Hormone-releasing Hormone (LHRH), Advanced Prostate Cancer
- Dosage Form: Intravenous injection
- Specification: 50 mg
Bicalutamide Tablets Scope of Application
Bicalutamide Tablets, in combination with luteinizing hormone-releasing hormone (LHRH) analogues or surgical orchiectomy for the treatment of advanced prostate cancer.
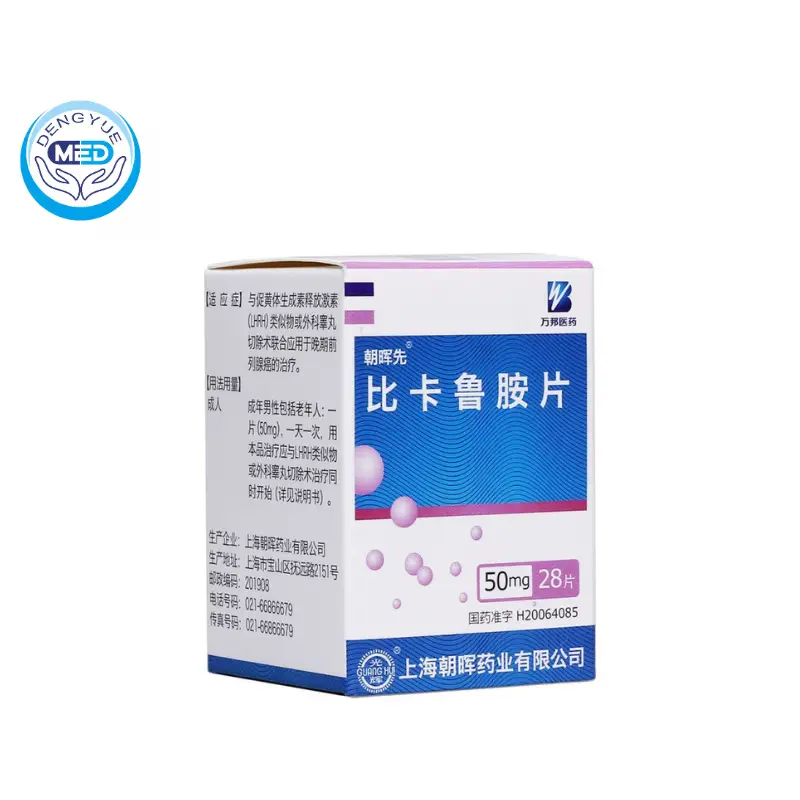
Bicalutamide Tablets Characteristics
Ingredients: Bicalutamide
Properties: white film-coated tablets, white color after removing the coating.
Specification: 50mg
Storage: Shielded from light and kept airtight.
Effective period: 24 months
Execution Standard: State Drug License H20064085
Approval Number: YBH05052006
Manufacturer: Shanghai Chaohui Pharmaceutical Co.
Bicalutamide Tablets Usage Guidelines
Bicalutamide TabletsDosage and Usage:
Adults: adult males including the elderly: one tablet (50mg) once a day, treatment with Comstock should be initiated at the same time as treatment with LHRH analogs or surgical orchiectomy.
CHILDREN: This product is contraindicated in children.
RENAL IMPAIRMENT: No dose adjustment is necessary for patients with renal impairment.
Hepatic impairment: No dose adjustment is necessary in patients with mild hepatic impairment; drug accumulation may occur in patients with moderate to severe hepatic impairment.
Bicalutamide Tabletsadverse reactions:
May cause flushing, scratching, additional breast tenderness and gynecomastia, which may diminish with orchiectomy.
It may also cause diarrhea, nausea, vomiting, malaise and dry skin.
Bicalutamide Tablet Medication Restrictions
Contraindications: contraindicated in women and children, can not be used in patients who are allergic to the product.
Precautions:
Comstock is extensively metabolized in the liver. Data suggest that drug clearance may be slowed in patients with severe hepatic impairment, which may lead to accumulation. Therefore, Comstock should be used with caution in patients with moderate to severe hepatic impairment. INTERACTIONS WITH OTHER DRUGS AND OTHER FORMS OF INTERACTION: There are no pharmacodynamic or pharmacokinetic interactions between Comstock and LHRH analogs. No interactions have been observed between Comstock and commonly prescribed drugs in combination.
Note: If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYueMed. It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.




Reviews
There are no reviews yet.