Fasenra(Benralizumab)|Eosinophilic Asthma|HongKong DengYue Medicine
- Generic Name/Brand Name: Benralizumab/Fansutra
- Indications: Eosinophilic Asthma
- Dosage Form: solutions
- Specification: 30 mg/mL × 1 dose
Benralizumab Application Scope
Benralizumab Injection is a monoclonal antibody used in the treatment of asthma and chronic obstructive pulmonary disease (COPD).
It is specifically used for patients with severe eosinophilic asthma, a subtype of asthma characterized by elevated eosinophil levels in the blood and lungs. It works by targeting the interleukin-5 receptor alpha (IL-5Rα), reducing eosinophil counts and thereby decreasing inflammation and improving symptoms.
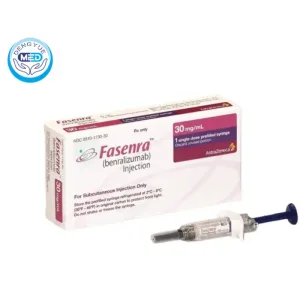
Benralizumab Injection Characteristics
-
Ingredients:
- Active Ingredient: Benralizumab (monoclonal antibody)
- Excipients: Common stabilizers and buffers used in the formulation for injection.
-
Properties:
- It is a humanized monoclonal antibody that binds to IL-5Rα on eosinophils, leading to their depletion via antibody-dependent cellular cytotoxicity (ADCC).
- It reduces the number of eosinophils in the blood and lung tissue, which helps control inflammation in diseases like asthma and eosinophilic granulomatosis with polyangiitis (EGPA).
-
Specification:
- Available as a 30 mg/1.5 mL solution for subcutaneous injection.
-
Packaging Specification:
- Prefilled syringe or autoinjector with a dose of 30 mg in 1.5 mL.
-
Storage:
- Store in the refrigerator at 2°C to 8°C (36°F to 46°F).
- Do not freeze. Protect from light.
-
Expiry Date:
- Refer to the packaging for the specific expiration date.
-
Executive Standard:
- Meets quality standards set by regulatory authorities like the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency).
-
Approval Number:
- FDA: BLA 761038 (for severe eosinophilic asthma treatment).
-
Date of Revision:
- Information updated periodically based on new clinical data or regulatory updates.
-
Manufacturer:
- AstraZeneca, in partnership with AbbVie, for the manufacturing and distribution of it.
Guidelines for the Use of Benralizumab for Asthma
- Indications:
- Severe eosinophilic asthma in adult and pediatric patients aged 12 years and older.
- Eosinophilic granulomatosis with polyangiitis (EGPA) as an add-on therapy.
Dosage and Administration
- Dosage:
- For severe eosinophilic asthma:
- Initial dosing: 30 mg subcutaneously every 4 weeks for the first 3 doses.
- Maintenance: After the initial dosing, administer 30 mg subcutaneously every 8 weeks.
- For EGPA:
- The dosage schedule may vary; typically, 30 mg subcutaneously every 4 weeks after the first three doses.
- For severe eosinophilic asthma:
- Administration:
- Administer via subcutaneous injection.
- It is important to train the patient or healthcare provider on the correct injection technique.
- Do not shake the vial. Allow the injection to reach room temperature before administering.
Benralizumab Side Effects
- Common Side Effects:
- Headache
- Pharyngitis (sore throat)
- Fever
- Injection site reactions (e.g., pain, redness)
- Serious Side Effects:
- Hypersensitivity reactions: Rash, urticaria, or anaphylaxis (rare)
- Infections: Respiratory tract infections, herpes zoster
Note: Benralizumab for Asthma may increase the risk of serious infections. Patients should be monitored closely for any signs of infection.
Contraindications
- Hypersensitivity: It should not be administered to patients with a known hypersensitivity to benralizumab Injection or any of its components.
Precautions
-
Infections:
- Patients should be monitored for signs and symptoms of infection, especially upper respiratory infections.
- It may be advisable to treat latent tuberculosis before starting it.
-
Parasitic Infections:
- As with any immunosuppressive therapy, caution should be used in patients with a history of parasitic infections.
-
Pregnancy and Lactation:
- The safety of it during pregnancy and lactation has not been established. It should only be used during pregnancy if the potential benefit justifies the potential risk.
Benralizumab Interactions
-
Drug Interactions:
- Currently, no significant drug-drug interactions have been reported with Benralizumab Asthma. However, caution is advised when combining with other immunosuppressive therapies, as this could increase the risk of infections.
Note: Always inform healthcare providers about all medications being taken, including over-the-counter drugs and herbal supplements.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from Hong Kong DengYue Medicine. It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.






Reviews
There are no reviews yet.