Belzutifan|Kidney Cancer|HongKong DengYue Medicine
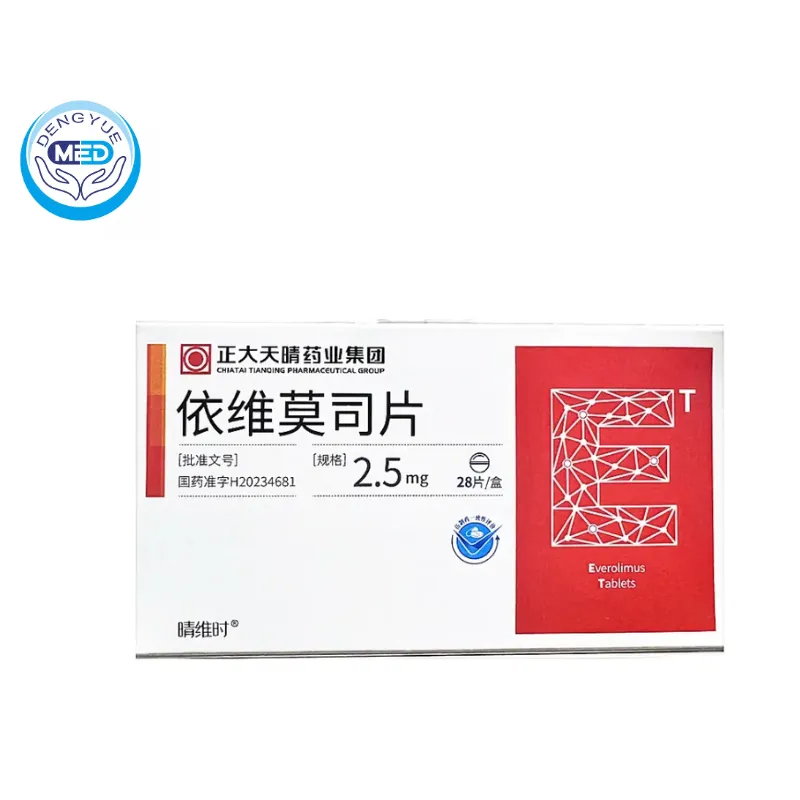
- Generic Name/Brand Name: Belzutifan/WELIREG
- Indications: Kidney Cancer
- Dosage Form: film-coated tablets
- Specification: 40 mg
Belzutifan Application Scope
Belzutifan, marketed under the brand name WELIREG®, is an oral hypoxia-inducible factor-2 alpha (HIF-2α) inhibitor.
It is indicated for the treatment of adult patients with von Hippel-Lindau (VHL) disease who require therapy for associated renal cell carcinoma (RCC), central nervous system (CNS) hemangioblastomas, or pancreatic neuroendocrine tumors (pNET) that do not require immediate surgery.

Belzutifan Package Insert
-
Ingredients: Welireg drug is the active pharmaceutical ingredient. Each WELIREG tablet contains 40 mg of belzutifan.
-
Properties: Belzutifan inhibits HIF-2α, a transcription factor involved in tumor growth and survival under low oxygen conditions. By inhibiting HIF-2α, Welireg drug disrupts processes that promote tumor growth.
-
Specification: WELIREG is supplied as 40 mg film-coated tablets for oral administration.
-
Packaging Specification: Specific packaging details are not provided in the available sources.
-
Storage: Store at controlled room temperature, 20°C to 25°C (68°F to 77°F).
-
Expiry Date: The shelf life or expiry date is not specified in the provided sources.
-
Executive Standard: WELIREG is approved by the U.S. Food and Drug Administration (FDA) for medical use.
-
Approval Number: The specific approval number is not mentioned in the available sources.
-
Date of Revision: The prescribing information was last updated in August 2021.
-
Manufacturer: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
Guidelines for the Use of Belzutifan
Dosage and Administration:
The recommended dosage of WELIREG is 120 mg (three 40 mg tablets) taken orally once daily, with or without food, at the same time each day. Treatment should continue until disease progression or unacceptable toxicity occurs.
Belzutifan Side Effects:
Common adverse reactions (≥20%) include decreased hemoglobin, anemia, fatigue, increased creatinine, headache, dizziness, increased glucose, and nausea.
Contraindications:
There are no listed contraindications for WELIREG.
Precautions:
- Embryo-Fetal Toxicity: Exposure to WELIREG during pregnancy can cause fetal harm. Verify pregnancy status before initiating treatment. Advise patients to use effective non-hormonal contraception, as WELIREG can render some hormonal contraceptives ineffective.
Belzutifan Interactions
Drug Interactions:
-
CYP2C19 and CYP3A4 Inhibitors: Co-administration with strong CYP2C19 and CYP3A4 inhibitors can increase Welireg drug exposure, potentially enhancing adverse reactions.
-
CYP2C19 and CYP3A4 Inducers: Co-administration with strong CYP2C19 and CYP3A4 inducers can decrease Welireg drug exposure, potentially reducing its efficacy.
-
Hormonal Contraceptives: Belzutifan can reduce the effectiveness of hormonal contraceptives. Advise patients to use effective non-hormonal contraception during treatment and for one week after the last dose.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from HongKong DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.



Reviews
There are no reviews yet.