Avastin (Bevacizumab) – mCRC & NSCLC | HongKong DengYue Medicine
- Generic Name/Brand Name: Bevacizumab / Avastin®
- Indications: mCRC, NSCLC, RCC, HCC, OvCa, CervCa
- Dosage Form: Injection
- Specification: 100 mg, 4 mL × 1 vial/box
Bevacizumab Application Scope
Bevacizumab is a recombinant humanized monoclonal antibody that selectively binds to vascular endothelial growth factor A (VEGF-A), inhibiting angiogenesis and thereby suppressing tumor growth and metastasis. It is widely used in combination with chemotherapy or targeted agents for metastatic colorectal cancer, unresectable locally advanced, recurrent, or metastatic non-squamous non-small cell lung cancer, metastatic renal cell carcinoma, recurrent glioblastoma (in some regions), epithelial ovarian, fallopian tube, and primary peritoneal cancers, as well as persistent, recurrent, or metastatic cervical cancer. In combination with atezolizumab, it is also indicated for unresectable hepatocellular carcinoma.
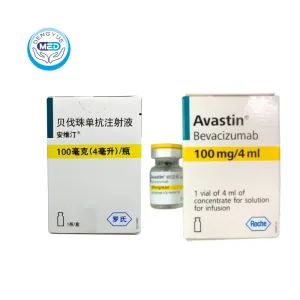
Bevacizumab Characteristics
-
Ingredients: Bevacizumab as active ingredient, with trehalose dihydrate, sodium phosphate, polysorbate 20, and water for injection.
-
Properties: Clear to slightly opalescent, colorless to pale brown sterile solution for intravenous infusion.
-
Packaging Specification: 100 mg, 4 mL × 1 vial/box;
-
Storage: Store at 2–8 °C in original carton; protect from light; do not freeze or shake.
-
Expiry Date: 24 months from manufacturing date; check carton for exact date.
-
Executive Standard: Approved under BLA or equivalent biologics registration per region.
-
Approval Number: Refer to local regulatory authority’s approval code (e.g., US FDA BLA 125085).
-
Date of Revision: Latest update per local prescribing information.
-
Manufacturer:
-
Genentech, Inc. (a member of the Roche Group).
-
Guidelines for the Use of Avastin
-
Dosage and Administration:
-
Administration:
-
Administer as IV infusion only.
-
First infusion over 90 minutes; if well tolerated, subsequent infusions may be shortened to 60 then 30 minutes.
-
Do not administer as IV push or bolus.
-
-
Missed Dose: Administer missed infusion as soon as possible, then continue on the regular schedule.
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
Hypertension
-
Proteinuria
-
Fatigue
-
Epistaxis and mucosal bleeding
-
Headache
-
Nausea, diarrhea, constipation
-
Wound-healing complications
-
-
Serious Adverse Reactions:
-
Gastrointestinal perforation or fistula
-
Severe hemorrhage or hemoptysis
-
Arterial and venous thromboembolism
-
Hypertensive crisis
-
Nephrotic syndrome, severe proteinuria
-
Reversible posterior leukoencephalopathy syndrome (RPLS)
-
Congestive heart failure, cardiac ischemia
-
-
-
Contraindications:
-
Hypersensitivity to bevacizumab or formulation components
-
Do not use in patients with recent hemoptysis or untreated CNS hemorrhage
-
-
Precautions:
-
Monitor blood pressure regularly and manage hypertension before and during treatment
-
Perform urine protein testing periodically
-
Delay or discontinue treatment for severe wound-healing complications or GI perforation
-
Avoid elective surgery during treatment; resume after adequate wound healing
-
Monitor for signs of bleeding, thrombosis, heart failure, or neurological symptoms
-
Bevacizumab Interactions
-
Anticoagulants/antiplatelets: May increase bleeding risk — monitor closely
-
Other anti-VEGF agents: Additive risk of toxicity — avoid combination
-
CYP interactions: Not expected, as bevacizumab is a monoclonal antibody
-
Pharmacodynamic interactions: Caution with drugs affecting wound healing or blood pressure
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.





Reviews
There are no reviews yet.