Augtyro Repotrectinib Capsules | Lung Cancer | DengYue
- Generic Name/Brand Name: Repotrectinib/Augtyro
- Indications: Non-small cell lung cancer (NSCLC) ,solid tumors
- Dosage Form: Capsules
- Specification: 40 mg, 160 mg
Augtyro Capsules Application Scope
Adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) that is ROS1-positive.
Adult and pediatric patients 12 years of age and older with solid tumors that have an NTRK gene fusion, are locally advanced or metastatic, and have progressed following treatment or have no satisfactory alternative therapy.
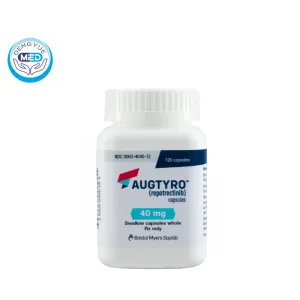
Augtyro Capsules Characteristics
-
Ingredients: Each capsule contains repotrectinib as the active ingredient.
-
Properties: Repotrectinib is a tyrosine kinase inhibitor targeting ROS1 and NTRK gene fusions.
-
Specification: Available in 40 mg and 160 mg capsules.
-
Packaging Specification: Supplied as hard-shell capsules containing white to off-white powder.
-
Storage: Store at room temperature, 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F to 86°F).
-
Expiry Date: Refer to the packaging for the expiration date.
-
Executive Standard: Complies with applicable pharmaceutical standards as approved by regulatory authorities.
-
Approval Number: Refer to the packaging or prescribing information for the approval number.
-
Date of Revision: Refer to the prescribing information for the date of the latest revision.
-
Manufacturer: Bristol Myers Squibb.
Guidelines For The Use Of Augtyro Capsules
Dosage and Administration:
For adult and pediatric patients 12 years and older:
Take 160 mg orally once daily with or without food for 14 days, then increase to 160 mg twice daily, continuing until disease progression or unacceptable toxicity.
Swallow capsules whole; do not open, chew, crush, or dissolve.
If a dose is missed or vomiting occurs after taking a dose, skip the dose and resume at the next scheduled time.
Adverse Reactions:
Common adverse reactions (≥20%) include dizziness, dysgeusia, peripheral neuropathy, constipation, dyspnea, fatigue, ataxia, cognitive impairment, muscular weakness, and nausea.
Serious adverse reactions may include interstitial lung disease/pneumonitis, hepatotoxicity, and central nervous system effects.
Contraindications: None.
Precautions:
Monitor for signs of central nervous system adverse reactions, hepatotoxicity, and interstitial lung disease/pneumonitis.
Advise patients not to drive or operate machinery if experiencing dizziness or other central nervous system effects.
Use effective non-hormonal contraception during treatment and for a specified period after the last dose, as Augtyro may reduce the effectiveness of hormonal contraceptives.
Augtyro Capsules Interactions
Drug Interactions:
-
Avoid concomitant use with strong or moderate CYP3A inhibitors and inducers, as they may affect repotrectinib exposure.
-
Avoid concomitant use with P-gp inhibitors.
-
Repotrectinib may decrease the concentration of CYP3A4 substrates, potentially reducing their efficacy.
-
Avoid concomitant use with hormonal contraceptives; advise the use of effective non-hormonal contraception.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.






Reviews
There are no reviews yet.