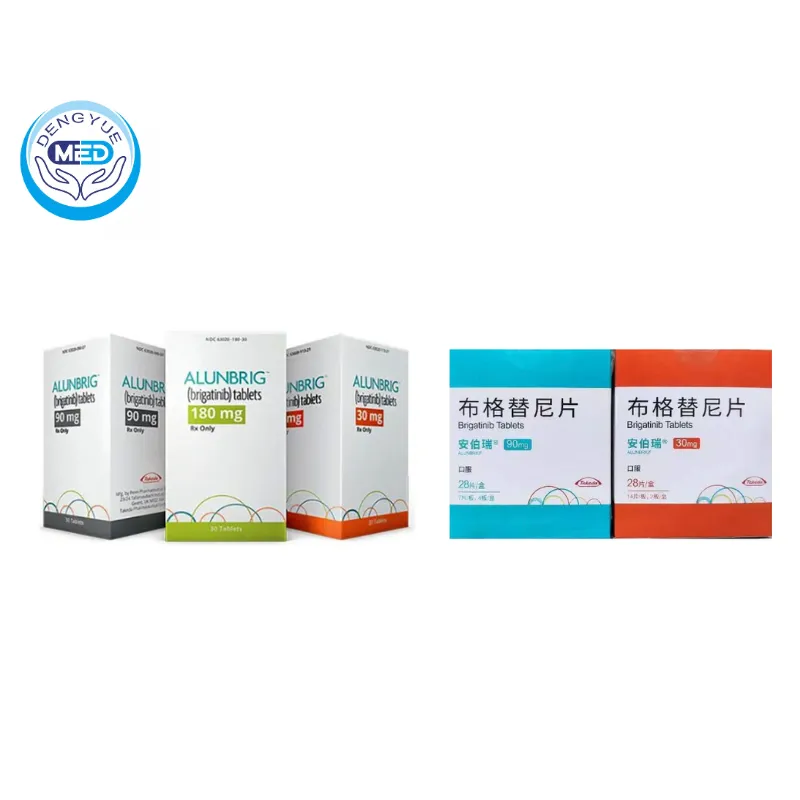
Alunbrig (Brigatinib) | NSCLC | HongKong DengYue Medicine
- Generic Name/Brand Name: Brigatinib/Alunbrig
- Indications: ALK-positive metastatic non‑small cell lung cancer (NSCLC)
- Dosage Form: Film-coated tablets, oral route.
- Specification: 30 mg, 90 mg, and 180 mg x 28 tablets
Brigatinib Application Scope
Treatment of adult patients with ALK-positive metastatic non‑small cell lung cancer (NSCLC), including those who have progressed on or are intolerant to crizotinib.

Brigatinib Characteristics
-
Ingredients:
- Active: Brigatinib (a small‑molecule ALK/ROS1 tyrosine kinase inhibitor)
-
Properties: Orally active kinase inhibitor targeting ALK, ROS1, IGF‑1R, FLT‑3, and select EGFR mutations
-
Packaging Specification: Film‑coated tablets: 30 mg (round, “U3”) & 90 mg (oval, “U7”)
-
Storage: Store at controlled room temperature: 20 °C to 25 °C (68–77 °F); excursions allowed between 15–30 °C
-
Expiry Date: As per printed package—refer to individual cartons; typical shelf‑life ~24 months from manufacture (check label for specifics).
-
Executive Standard: Follows FDA-approved prescribing information (PIs) and USP standards.
-
Approval Number: NDA 208772 (U.S. FDA); marketed under brand name Alunbrig®.
-
Date of Revision: Most recent label revision accessed in 2024–2025 (label PDF July 2024)
-
Manufacturer: Originally Ariad Pharmaceuticals, now Takeda Pharmaceutical Company Limited
Guidelines for the Use of Brigatinib
-
Dosage and Administration:
-
Initial: 90 mg orally once daily for 7 days.
-
If tolerated, escalate to 180 mg once daily (maintenance dose)
-
-
Adverse Reactions:
- Common AEs (≥25%): diarrhea, fatigue, nausea, rash, cough, myalgia, headache, hypertension, vomiting, dyspnea
Serious/fatal events: interstitial lung disease/pneumonitis (3–6%), pneumonia, pulmonary embolism, respiratory failure, sudden death (~3.7%)
- Common AEs (≥25%): diarrhea, fatigue, nausea, rash, cough, myalgia, headache, hypertension, vomiting, dyspnea
-
Contraindications: Unknown.
-
Precautions:
-
Monitor pulmonary symptoms especially first week
-
Monitor blood pressure, heart rate (bradycardia), pancreas enzymes, blood glucose, CPK, liver function
-
Fetal risk: causes embryo‑fetal toxicity; advise contraception (females: non‑hormonal during treatment + 4 months; males: partners use contraception during + 3 months)
-
Interactions
- Drug Interactions:
-
CYP3A inducers (e.g., rifampin, carbamazepine, phenytoin): decrease brigatinib exposure; avoid or increase brigatinib dose
-
CYP3A inhibitors (e.g., ketoconazole, clarithromycin): increase exposure; avoid or reduce dose ~50% .
-
Brigatinib induces CYP3A → may reduce effectiveness of hormonal contraceptives, midazolam, ethosuximide, etc.; use non‑hormonal birth control
-
Apart from CYP interactions, severe interactions noted with drugs like doravirine
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.








Reviews
There are no reviews yet.