Aclidinium Bromide|Pulmonary Disease (PD)|HongKong DengYue Medicine
- Generic Name/Brand Name: Aclidinium Bromide/Covis Pharma
- Indications: Chronic Obstructive Pulmonary Disease (COPD)
- Dosage Form: Multi-dose dry powder
- Specification: 400 mcg
Aclidinium Bromide Application Scope
Aclidinium bromide is a long-acting muscarinic antagonist (LAMA) used primarily for the maintenance treatment of chronic obstructive pulmonary disease (COPD).
It works by inhibiting the M3 muscarinic receptor in airway smooth muscle, leading to bronchodilation and improved airflow.
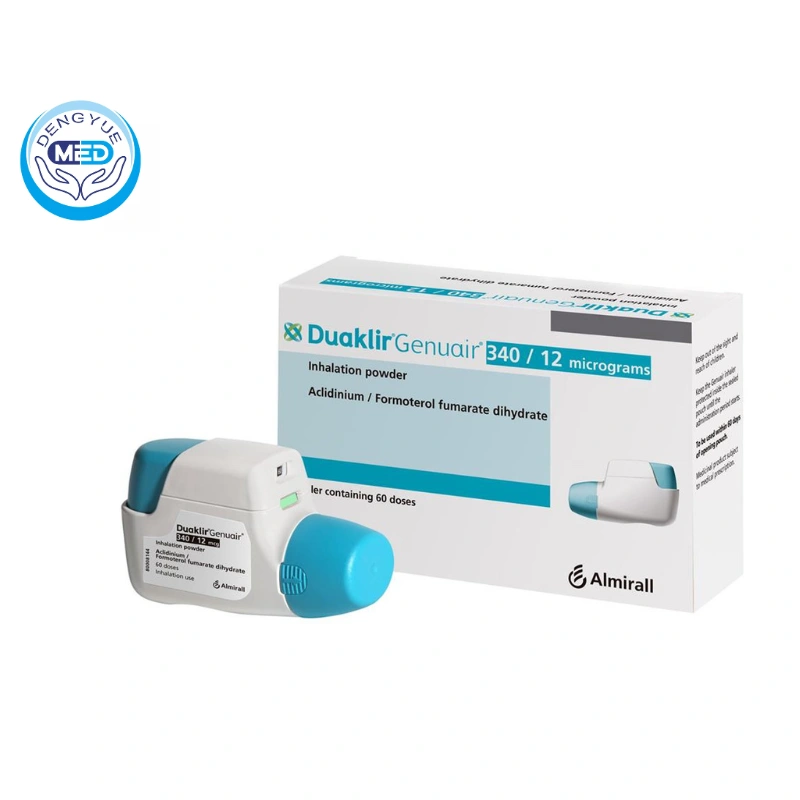
Characteristics of Aclidinium Bromide
-
Ingredients: Aclidinium bromide is the active ingredient, often combined with formoterol fumarate in certain formulations.
-
Properties: As a LAMA, aclidinium provides bronchodilation by blocking muscarinic receptors in the lungs, reducing bronchospasm associated with COPD.
-
Specification: Each actuation of the inhaler delivers 400 mcg of aclidinium.
-
Packaging Specification: Aclidinium is supplied as a breath-actuated, multi-dose dry powder inhaler.
-
Storage: Store at room temperature, away from moisture and heat.
-
Expiry Date: Refer to the packaging for the specific expiration date.
-
Executive Standard: Approved by regulatory authorities such as the FDA.
-
Approval Number: Refer to the specific product labeling for approval details.
-
Date of Revision: Check the prescribing information for the latest revision date.
-
Manufacturer: Various manufacturers produce aclidinium bromide under different brand names, including TUDORZA® PRESSAIR®.
Guidelines for the Use of Aclidinium Bromide
-
Dosage and Administration: The recommended dose is one oral inhalation of 400 mcg twice daily, approximately 12 hours apart.
-
Adverse Reactions: Common side effects include headache, nasopharyngitis, and cough. Serious adverse reactions are rare but may include paradoxical bronchospasm.
-
Contraindications: Patients with severe hypersensitivity to milk proteins or any component of the product should not use aclidinium.
-
Precautions: Use with caution in patients with narrow-angle glaucoma, urinary retention, or severe hypersensitivity to atropine or its derivatives.
Aclidinium Bromide Interactions
- Drug Interactions: No formal studies have been conducted, but concomitant use with other anticholinergic-containing drugs may increase the risk of adverse effects.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from HongKong DengYue Medicine. It is only for internal discussion among medical staff and does not serve as a basis for medication.
- For specific medication guidelines, please consult the attending physician.









Reviews
There are no reviews yet.