
What Is Qipani Olaparib Used For? China Approves First Domestic Olaparib Generic, Marking a New Milestone in PARP Inhibitor Access
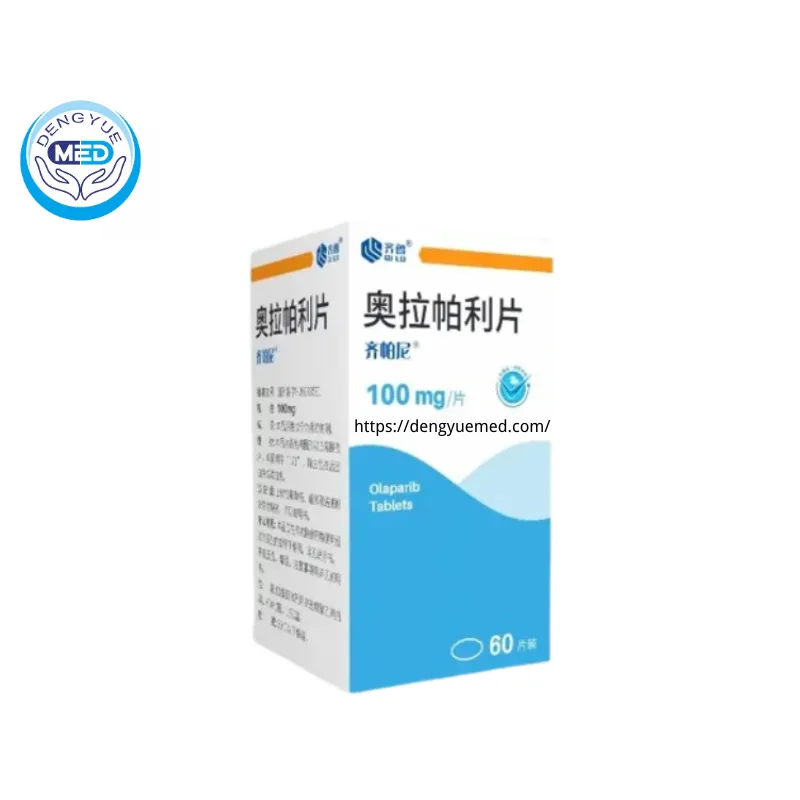
In May 2023, China’s National Medical Products Administration (NMPA) approved the first domestically developed generic version of olaparib tablets. Developed by Qilu Pharmaceutical, this olaparib product was officially approved on May 12 and subsequently launched under the brand name Qipani®.
👉 As the first domestic olaparib generic approved in China, Qipani® represents an important milestone in the local availability of PARP inhibitor therapies.
Olaparib is a well-established targeted therapy with demonstrated clinical value across multiple solid tumors, particularly in patients with defects in DNA damage repair pathways.
The approval and commercialization of Qipani® reflect China’s continued efforts to improve access to innovative cancer treatments by introducing high-quality, regulatory-approved generic medicines. For patients requiring long-term maintenance therapy, broader availability may translate into improved treatment continuity and adherence.
🤔 Against this background, a key question for clinicians, patients, and caregivers remains: what is Qipani olaparib used for, and how does it fit into modern cancer treatment strategies?
What Is Qipani Olaparib Used For?
What is Qipani olaparib used for? Qipani® contains olaparib, a poly(ADP-ribose) polymerase (PARP) inhibitor used in the treatment of selected cancers characterized by defects in DNA repair mechanisms.
Olaparib tablets are primarily indicated for patients whose tumors harbor mutations in genes involved in homologous recombination repair (HRR), most notably BRCA1 and BRCA2, or who demonstrate homologous recombination deficiency (HRD).
In clinical practice, olaparib is commonly used as:
- Maintenance therapy following a response to platinum-based chemotherapy, particularly in ovarian cancer
- Targeted treatment for advanced or metastatic cancers with specific genetic alterations
Qipani® is approved based on the same therapeutic principles as reference Lynparza (Olaparib), and its clinical use follows the approved indications established by regulatory authorities in China.
✨ As a PARP inhibitor, olaparib does not act through non-specific cytotoxic effects but instead targets a defined molecular vulnerability within cancer cells, making it a key component of precision oncology.
How Does Qipani Olaparib Work as a PARP Inhibitor?
To understand what Qipani olaparib is used for, it is essential to examine how olaparib works at the molecular level.
PARP enzymes, particularly PARP-1 and PARP-2, play a central role in the repair of single-strand DNA breaks through the base excision repair pathway. Under normal conditions, these enzymes help maintain genomic stability by detecting DNA damage and coordinating repair processes.
In tumors with BRCA1 or BRCA2 mutations, the homologous recombination repair pathway—responsible for repairing double-strand DNA breaks—is impaired. As a result, cancer cells become increasingly dependent on alternative repair mechanisms, including PARP-mediated pathways, to survive.
🎯 Olaparib inhibits PARP activity, preventing the repair of single-strand DNA breaks. During DNA replication, these unrepaired lesions can evolve into double-strand breaks. In cells already deficient in homologous recombination, such damage becomes irreparable, ultimately leading to cancer cell death. This phenomenon is known as synthetic lethality.
By exploiting this specific vulnerability, Qipani olaparib selectively targets tumor cells with DNA repair defects while largely sparing normal cells, which retain intact repair pathways. This targeted mechanism underpins both the efficacy and the generally manageable safety profile of PARP inhibitors.
Approved Indications of Olaparib in China
An important part of answering what is Qipani olaparib used for lies in understanding its approved indications in China. According to NMPA-approved labeling, olaparib is indicated for use in several cancer types under defined clinical conditions.
Ovarian, Fallopian Tube, and Primary Peritoneal Cancer
Olaparib is approved for adult patients with advanced epithelial ovarian cancer, fallopian tube cancer, or primary peritoneal cancer in the following settings:
- Patients with germline or somatic BRCA mutations who achieve complete or partial response after first-line platinum-based chemotherapy, as maintenance treatment
- Patients with HRD-positive tumors who respond to first-line platinum-based chemotherapy combined with bevacizumab, as maintenance therapy
- Patients with platinum-sensitive recurrent disease who achieve response after platinum-based chemotherapy, as maintenance treatment
Prostate Cancer
Olaparib is also approved for the treatment of adult patients with metastatic castration-resistant prostate cancer (mCRPC) who carry germline or somatic BRCA mutations and have experienced disease progression following prior treatment, including at least one novel hormonal agent.
These approved indications reflect a growing recognition of the role of DNA repair defects in tumor biology and highlight the importance of genetic testing in treatment decision-making.
China Approval of the First Domestic Olaparib Generic: Qipani®
The approval of Qipani® marks the first time a domestically developed olaparib generic has entered the Chinese market. This approval was granted following rigorous regulatory evaluation to ensure quality consistency, bioequivalence, and therapeutic reliability in line with national standards.
Qipani® is available in multiple tablet strengths, allowing for flexible dosing adjustments in clinical practice. This is particularly relevant for patients receiving long-term maintenance therapy, where dose modification may be necessary to manage tolerability while maintaining therapeutic benefit.
By expanding the availability of olaparib within China, Qipani® supports broader patient access to PARP inhibitor therapy and aligns with ongoing efforts to integrate targeted treatments into standard oncology care pathways.
Clinical and Patient Impact
From a clinical perspective, the introduction of Qipani® may help address practical challenges associated with long-term cancer treatment. Maintenance therapies such as olaparib often require sustained administration over extended periods, making treatment continuity an important consideration.
Improved access to approved generic options has the potential to:
- Reduce barriers to initiation and continuation of therapy
- Support adherence to guideline-recommended treatment durations
- Enable more patients to benefit from precision oncology strategies
For clinicians, the availability of a domestically approved olaparib product offers additional flexibility when designing individualized treatment plans based on molecular characteristics, disease stage, and patient tolerance.
Future Perspectives for Olaparib and PARP Inhibition
Beyond current indications, ongoing research continues to explore the role of PARP inhibitors in combination strategies and in tumors with broader DNA repair deficiencies. Studies are evaluating combinations of olaparib with hormonal therapies, immunotherapies, and other targeted agents to enhance efficacy and overcome resistance.
As understanding of tumor genomics evolves, the clinical utility of PARP inhibitors such as olaparib may continue to expand. In this context, the availability of high-quality, approved domestic products like Qipani® provides a foundation for sustainable integration of these therapies into routine cancer care.
Conclusion
In summary, what is Qipani olaparib used for can be clearly defined within the framework of modern precision oncology. Qipani® is used for the treatment and maintenance therapy of selected cancers—most notably ovarian cancer and metastatic castration-resistant prostate cancer—in patients with specific DNA repair deficiencies.
The China approval of Qipani®, the first domestic olaparib generic, represents a meaningful step toward improving access to PARP inhibitor therapy. As targeted treatments continue to reshape cancer management, such approvals play an important role in ensuring that advances in molecular medicine translate into real-world patient benefit.



