
Novel Hemophilia Treatment Turoctocog Alfa Pegol Approved By FDA, Bringing New Hope To Patients
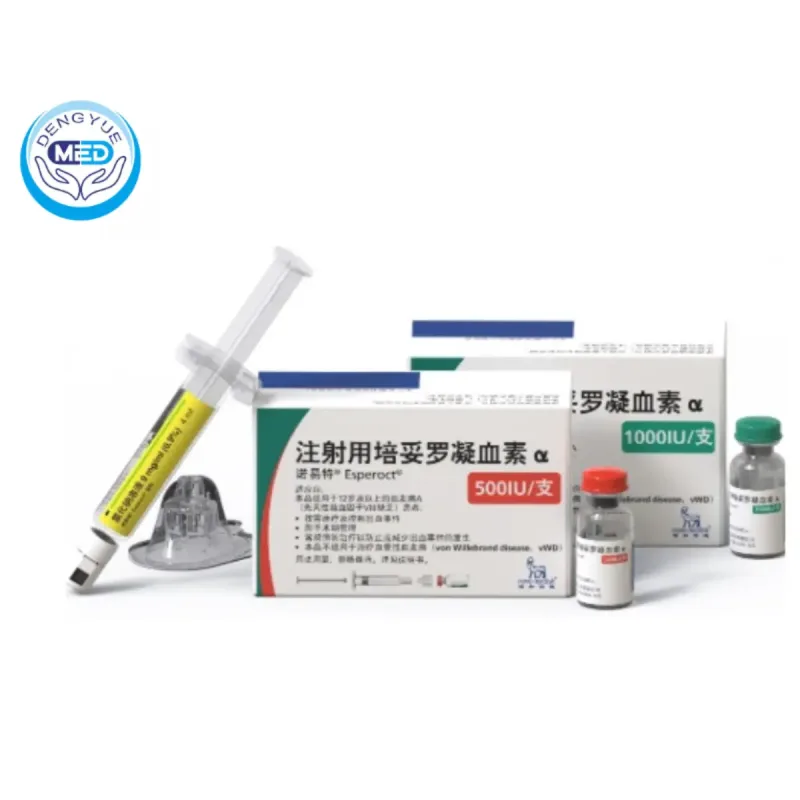
Recently, the U.S. Food and Drug Administration (FDA) officially approved Turoctocog Alfa Pegol (brand name: Esperoct®), a new long-acting Factor VIII drug developed by Novo Nordisk, for the treatment of Hemophilia A.
This groundbreaking drug offers a more convenient and effective treatment option for hemophilia patients worldwide.
What Is Turoctocog Alfa Pegol?
Turoctocog Alfa is a recombinant Factor VIII (rFVIII) drug modified through PEGylation, which extends its half-life in the body.
This means patients can reduce the frequency of injections while maintaining stable Factor VIII levels, thereby better preventing bleeding episodes.
Hemophilia A is an inherited bleeding disorder caused by a deficiency or dysfunction of Factor VIII.
Patients typically require regular Factor VIII injections to prevent spontaneous bleeding, especially in joints, muscles, and internal organs.
Traditional Factor VIII drugs require frequent injections (usually 2-3 times per week), whereas the extended half-life of Turoctocog Alfa Pegol allows patients to inject just once a week, significantly improving treatment convenience and quality of life.
Turoctocog Alfa Pegol Clinical Trials
The approval of Turoctocog Alfa is based on data from multiple clinical trials, including global multicenter Phase III trials.
The results demonstrated that the drug is highly effective in preventing and treating bleeding episodes, maintaining patients’ Factor VIII levels within a safe range. Additionally, its safety has been thoroughly validated, with no serious side effects or immunogenicity issues identified.
The Chief Medical Officer of Novo Nordisk stated, “Turoctocog Alfa Pegol approval is a significant milestone in the field of hemophilia treatment.
We believe this innovative drug will provide patients with a more flexible treatment option, helping them better manage their condition, reduce bleeding risks, and improve their daily quality of life.”
Expectations From Patients And Doctors
For hemophilia patients and their families, the approval of Turoctocog Alfa Pegol is undoubtedly exciting news.
Many patients expressed that reducing injection frequency not only alleviates physical burdens but also reduces psychological stress, enabling them to participate in daily activities with greater confidence.
Dr. John Smith, a hemophilia specialist, noted, “The advent of long-acting Factor VIII drugs has revolutionized hemophilia treatment.
The introduction of Turoctocog Alfa Pegol will further optimize treatment regimens for patients, especially for those who require frequent injections. This is undoubtedly a major step forward.”
Future Prospects
With the launch of Turoctocog Alfa, the field of turoctocog alfa pegol hemophilia treatment has entered a new era.
Novo Nordisk stated that they will continue to dedicate efforts to the research and development of hemophilia drugs and explore more innovative therapies to further enhance patients’ quality of life.
Moreover, the approval of Turoctocog Alfa Pegol provides new insights for the treatment of other rare blood disorders.
In the future, with the development and launch of more long-acting drugs, patients with rare diseases are expected to benefit from more personalized and effective treatment options.
Conclusion
The approval of Turoctocog Alfa Pegol marks a new era in hemophilia treatment. This innovative drug not only offers patients a more convenient treatment option but also provides new research directions for the medical community.
We look forward to continued advancements in science and technology, bringing new hope to more patients with rare diseases.
However, DengYue’s efforts to increase the affordability of the medicine are critical to ensuring that more patients benefit from it.
We welcome you to reach out to us to get Turoctocog Alfa Pegol price. DengYueMedicine is here to provide detailed medicine information, pricing, and support to ensure a smooth and reliable buying experience.
Feel free to contact us anytime to discuss your needs or ask any questions about the medicine.
Information from DengYueMed, China Drug Import and Export Wholesaler offers this information as a guide, not a substitute for professional medical advice. Consult your doctor before any treatment.



