
TPIAO Approved for a New Indication: Clinical Progress of Recombinant Human Thrombopoietin in the Treatment of Thrombocytopenia
(China, December 23, 2025) — Sunshine Guojian Pharmaceutical, a leading biopharmaceutical company in China, today announced that TPIAO approved for a new indication—its independently developed Class 1 innovative drug—by the National Medical Products Administration (NMPA).
The newly approved indication is for the treatment of adult patients with chronic liver disease–associated thrombocytopenia (CLDT) who are scheduled to undergo surgical or diagnostic procedures.
✨ This approval marks TPIAO’s official expansion into the liver disease field—home to a large patient population—following its established use in oncology and hematology, offering an innovative therapeutic option with a clearly defined mechanism of action to address a long-standing clinical challenge.
Against this backdrop, the new indication has drawn further attention to comprehensive discussions on the drug’s mechanism of action, clinical value, and previously approved indications—topics that continue to be closely followed by pharmaceutical distribution and supply-chain partners, including DengyueMed.
Clinical Pain Point: Thrombocytopenia as the Primary Barrier to Liver Disease Surgery
Chronic liver disease (CLD) represents a major public health burden in China, with thrombocytopenia being one of its most common and challenging complications.
Data indicate that the prevalence of chronic liver disease–associated thrombocytopenia (CLDT) is approximately 6% among patients with non-cirrhotic chronic liver disease, rising to as high as 78% in patients with liver cirrhosis.
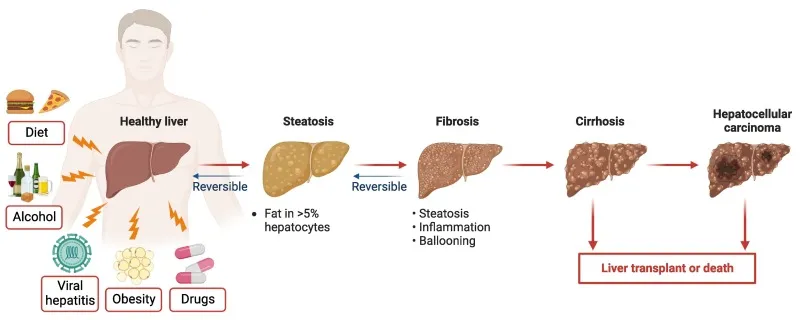
❗ As platelets (defined as a platelet count <100 × 10⁹/L) are critical components of hemostasis, severe thrombocytopenia substantially increases the risk of bleeding during essential diagnostic or therapeutic procedures, including liver biopsy, radiofrequency ablation, partial hepatectomy, and even liver transplantation.
In many cases, these procedures must be delayed or cancelled altogether.
For a long time, clinical management of such patients has relied primarily on a strategy of “passive replacement,” namely platelet transfusion.
However, this approach is constrained by multiple limitations, including shortages in platelet supply, the risk of transfusion-related infections, immune reactions, and the transient nature of its efficacy, which typically lasts only 1–3 days.
👉 Consequently, how to safely, effectively, and sustainably elevate platelet counts to meet surgical safety thresholds has remained a significant challenge for hepatologists, hepatobiliary surgeons, and interventional physicians alike.
Consequently, a paradigm shift is emerging in clinical practice toward production-stimulating therapeutic approaches. 🔻
TPIAO Approved for a New Indication: From “Replacement” to “Production-Stimulating” Therapy
The clinical value of TPIAO® in this newly approved indication stems from the high concordance between its mechanism of action and the pathophysiological characteristics of the disease, representing a paradigm shift from “passive replacement” to “active stimulation” therapy.
From a pathophysiological perspective, chronic liver disease–associated thrombocytopenia (CLDT) has a multifactorial etiology.
However, growing evidence in recent years has identified insufficient thrombopoietin (TPO) production due to impaired hepatic synthetic function as a central driving factor.
In healthy individuals, the liver produces the vast majority of endogenous TPO on a daily basis, whereas progressive liver diseases such as cirrhosis can reduce TPO production by as much as 80%.

👉 This distinctive pathological feature creates a precise therapeutic window for TPO replacement therapy.
As a full-length, fully human recombinant TPO, TPIAO is a recombinant human thrombopoietin (rhTPO) with an amino acid sequence that is 100% identical to endogenous human TPO.
By selectively binding to the c-Mpl receptor on the surface of bone marrow megakaryocyte progenitor cells, it activates key signaling pathways such as JAK2/STAT5, resulting in three major biological effects:
- Stimulation of megakaryocyte progenitor proliferation
- Acceleration of megakaryocyte differentiation and maturation
- Enhancement of platelet yield per individual megakaryocyte
✨ Through this mechanism, TPIAO not only increases platelet counts but also preserves the functional integrity of newly generated platelets.
Notably, the pharmacokinetic profile of TPIAO makes it particularly well suited for perioperative use.
✅ Following a single administration, platelet counts begin to rise significantly within 5–7 days, reach a peak at 10–14 days, and can be maintained at safe levels for 2–3 weeks.
This predictable and sustained platelet response provides surgeons with a clearly defined preoperative preparation window, enabling more reliable planning of elective procedures.
💪 Compared with conventional platelet transfusion, this endogenous platelet-stimulating mechanism avoids issues such as alloimmunization, transfusion-related infection risks, and platelet transfusion refractoriness (PTR).
In addition, by reducing reliance on allogeneic blood products, TPIAO offers broader public health benefits, including alleviating blood supply shortages and optimizing healthcare resource utilization.
This “treating the root cause” approach, in contrast to the “symptom-based” nature of platelet transfusion, allows platelet counts to be maintained at safe levels for a longer duration, provides greater flexibility in surgical scheduling, and fundamentally reshapes perioperative management strategies for patients with CLDT.
A Comprehensive Treatment Landscape: Building a Full-Course Management Matrix for Thrombocytopenia
The approval of the CLDT indication marks TPIAO approved for a new indication, representing TPIAO®’s successful expansion into a third major therapeutic area.
This milestone is not an isolated breakthrough but rather the result of a well-established body of clinical evidence and long-standing leadership in the thrombocytopenia treatment space.
🌐 As a benchmark product in platelet-raising therapy in China, Recombinant Human Thrombopoietin Injection (TPIAO) has now established a full-course disease management matrix spanning oncology, hematology, and liver disease, highlighting the strong platform value of rhTPO-based therapy.
In The Field of Oncology Supportive Care
TPIAO® has demonstrated well-validated efficacy in the treatment of chemotherapy-induced thrombocytopenia (CIT).
🔬 Clinical studies show that it can reduce the incidence of severe thrombocytopenia by approximately 50% and shorten the duration of grade III/IV thrombocytopenia by 3–5 days
More importantly, rhTPO helps maintain the relative dose intensity of chemotherapy at above 85%, thereby preserving the integrity and continuity of anticancer treatment.
As a result, it has been incorporated into several authoritative guidelines, including the Chinese Guidelines for the Diagnosis and Treatment of Chemotherapy-Induced Thrombocytopenia, and is recognized as a cornerstone therapy in oncology supportive care.
These achievements further reinforce the clinical significance of Recombinant Human Thrombopoietin Injection approval in this setting.
In The Hematology Arena
Tebio® has established a pivotal role in the treatment of primary immune thrombocytopenia (ITP).
🔬 Clinical data indicate an overall response rate of up to 85% in adult ITP patients, with a median time to response of only 7 days.
🔬 The approval of the pediatric indication in 2024 addressed a critical unmet need: a phase III study in children aged 6–17 years showed an overall response rate of 78.6% in the TPIAO® group, significantly superior to the control group, with a favorable safety profile.
On the strength of these results, TPIAO® has been listed for consecutive years as a preferred second-line treatment in the Chinese Guidelines for the Diagnosis and Treatment of Adult Primary Immune Thrombocytopenia.
These outcomes further illustrate the robustness of TPIAO’s mechanism of action as a recombinant human thrombopoietin therapy.
Taken together, TPIAO® has established a complete product lifecycle strategy—ranging from supportive care in oncology to disease-modifying treatment in hematology and now to perioperative management of chronic liver disease–associated thrombocytopenia.
Each expansion of indication has been driven by clearly defined clinical needs, robust evidence-based data, and differentiated therapeutic value.
Strategic Significance and Future Outlook
Dr. Lou Jing, Chairman and Chief Executive Officer of Sunshine Guojian Pharmaceutical, stated
“China has a large population of patients with liver disease, many of whom are unable to undergo necessary surgical procedures in a timely manner due to thrombocytopenia.
The successful expansion of TPIAO® into the liver disease field represents another important achievement in our continued focus on major unmet clinical needs.
We believe that this mechanistically innovative therapy will equip hepatologists with a powerful new tool and deliver tangible surgical opportunities and therapeutic benefits to a broad patient population.”
The approval of the CLDT indication carries dual strategic significance.
- 🛏️ From a clinical perspective, TPIAO® offers patients with chronic liver disease a predictable and sustainable ‘active platelet-boosting’ solution, fundamentally reshaping perioperative management paradigms for this population.
- ⌛ From a product lifecycle standpoint, this milestone marks TPIAO®’s successful expansion from oncology and hematologic disorders into the management of chronic diseases with a much larger patient base, completing its strategic positioning within the thrombocytopenia treatment landscape.
As a global pharmaceutical supplier, DengYue Pharma will leverage its nationwide cold-chain logistics network and hospital access capabilities to ensure the timely supply and professional support of TPIAO® for its newly approved indication across healthcare institutions at all levels.
🌐 This latest expansion further demonstrates that TPIAO® has moved beyond the traditional role of a “platelet-raising agent” to become a platform therapy capable of addressing multidisciplinary clinical challenges.
🙂 By continuously deepening its value across diverse disease areas, TPIAO® is helping to redefine standards of care for thrombocytopenia in China and providing a cornerstone for the development of a more comprehensive blood health management system.



