
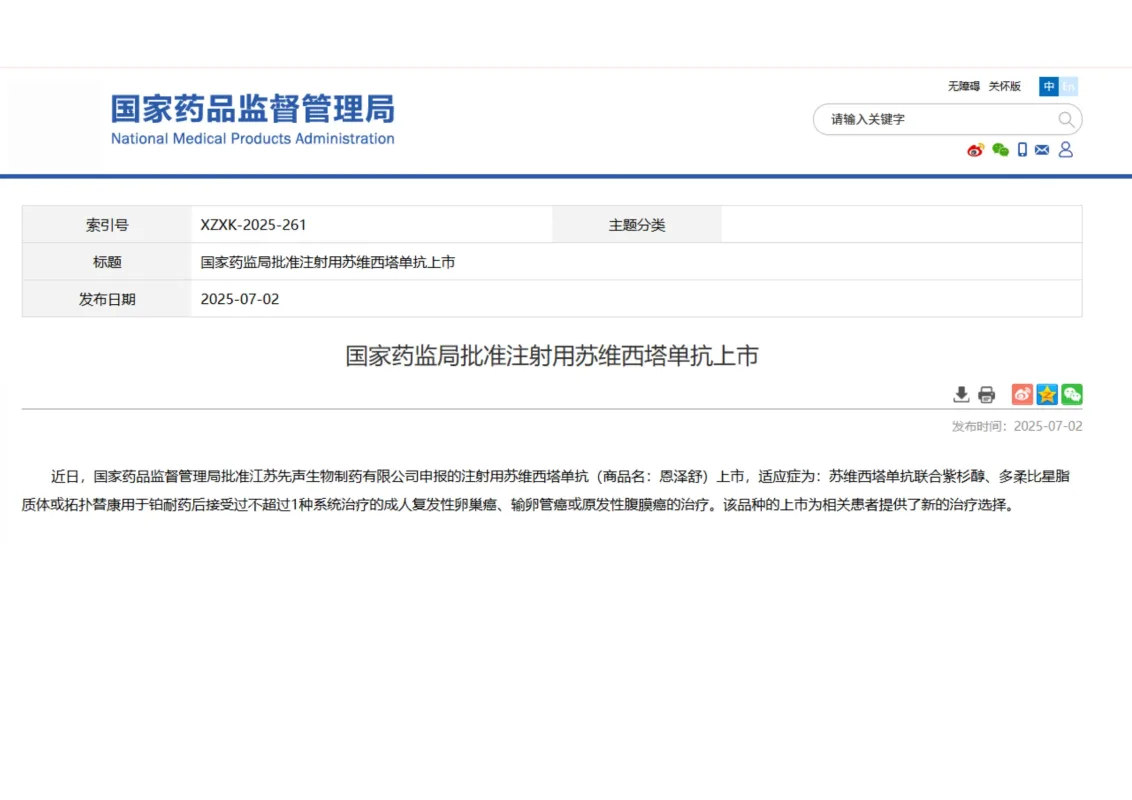
Suvemcitug Injection Approval in China丨Brings a New Treatment Option for Platinum-Resistant Ovarian Cancer
On July 3, 2025, Simcere Pharmaceutical Group Limited’s innovative drug subsidiary, Simcere, announced that its next-generation VEGF-targeting antibody, Suvemcitug Injection, had received marketing approval from the National Medical Products Administration (NMPA).
Suvemcitug is the first targeted therapy in China covering the entire population of patients with platinum-resistant ovarian cancer, filling a critical clinical gap.
💊 The drug is approved for use in combination with paclitaxel, pegylated liposomal doxorubicin, or topotecan in adult patients with recurrent ovarian, fallopian tube, or primary peritoneal cancer.
👉 They are platinum-resistant and have received no more than one prior systemic therapy.
Suvemcitug Injection’s Mechanism and Clinical Evidence
Suvemcitug is a recombinant humanized anti-VEGF rabbit monoclonal antibody designed to selectively bind to VEGF and block its biological activity.
🙅♂️ By inhibiting tumor angiogenesis at its source, the drug suppresses tumor growth and complements standard chemotherapy.
The SCORES Phase III clinical trial (NCT04908787) enrolled 421 patients with platinum-resistant recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who had failed prior platinum-based chemotherapy.
📈 Data from the trial demonstrated that median progression-free survival (PFS) reached 5.49 months in the Suvemcitug group, compared with 2.73 months in the placebo group (HR 0.46, P<0.0001).
✅ The objective response rate (ORR) was 26.0% versus 12.1%, according to blinded independent review committee assessment, confirming the drug’s significant clinical efficacy.
Professor Wu Lingying from the Chinese Academy of Medical Sciences Cancer Hospital, the lead investigator of the trial, remarked:
“The SCORES study is the first to demonstrate that vascular-targeted therapy can provide an overall survival benefit in platinum-resistant ovarian cancer. Suvemcitug’s approval addresses a long-standing clinical gap and is expected to reshape the anti-angiogenic treatment landscape.”
Patient Impact and Market Significance
😟 Ovarian cancer remains the leading cause of death among gynecologic malignancies in China, with approximately 61,100 new cases and 32,600 deaths annually (2024, National Cancer Center).
Fallopian tube cancer and primary peritoneal cancer share similar biological characteristics and are typically treated similarly.
👉Current standard treatments include surgery combined with platinum-based chemotherapy ± targeted maintenance therapy, but most patients relapse and develop platinum resistance, leaving very limited treatment options.
🙂 The approval of the Suvemcitug injection provides a new therapeutic option for patients who previously had few effective treatments, offering renewed hope for improved outcomes and quality of life.
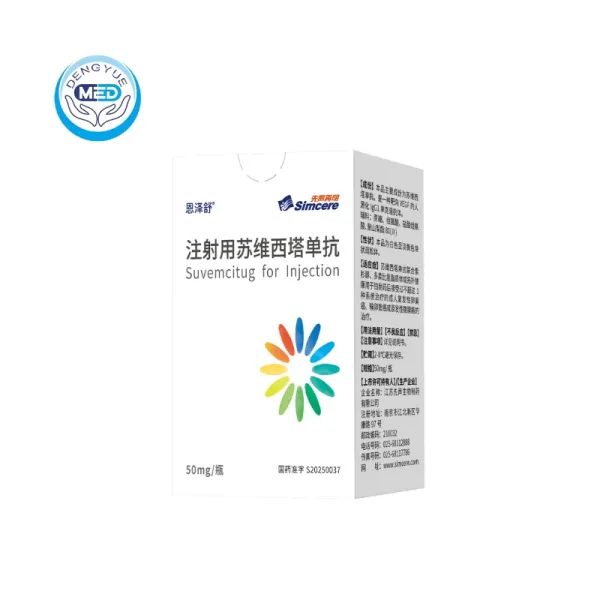
Enzeshu (Suvemcitug) – Ovarian | HongKong DengYue Medicine
- Generic Name/Brand Name: Suvemcitug / Enzeshu®
- Indications: Ovarian Cancer
- Dosage Form: Injection
- Specification: 50 mg / vial
✅ Hong Kong DengYue Medicine will continue to closely monitor developments in treatment options for ovarian, fallopian tube, and primary peritoneal cancers
✅ And we are committed to bringing more innovative Chinese drugs to the international market, providing safe and reliable new treatment options for patients worldwide.



