
Qalsody: A Milestone In The Treatment Of Acromegaly
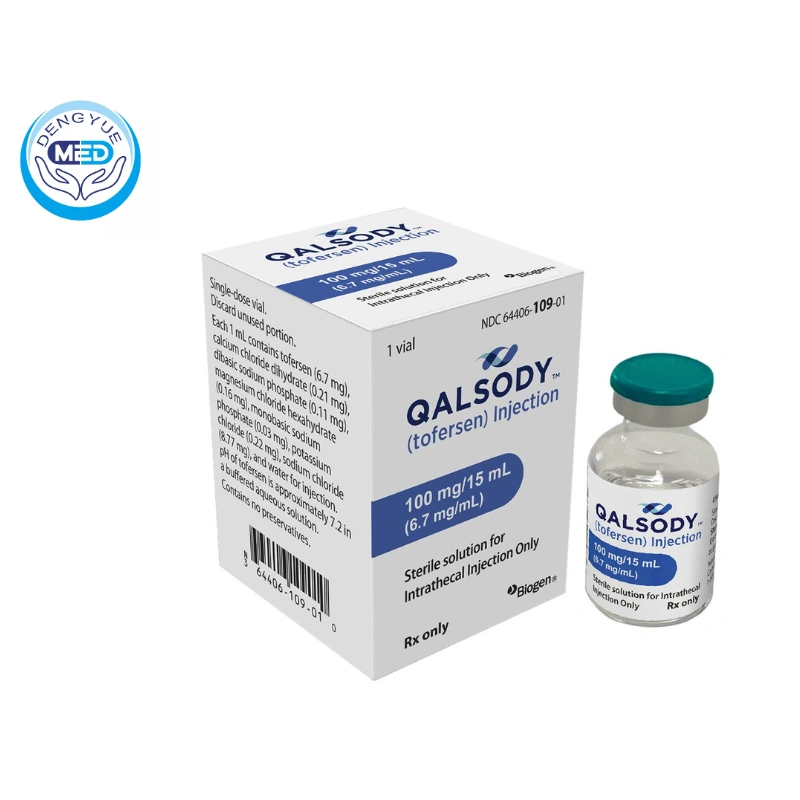
April 2023 – The U.S. Food and Drug Administration (FDA) today approved Qalsody (tofersen) for the treatment of amyotrophic lateral sclerosis (ALS) caused by mutations in the superoxide dismutase 1 (SOD1) gene.
This approval brings new treatment options to patients with this rare and fatal neurological disease.
About ALS And SOD1 Mutation
ALS, commonly known as “acromegaly,” is a progressive neurodegenerative disease that causes muscle weakness and atrophy, ultimately affecting respiratory function. Mutations in the SOD1 gene are responsible for approximately 2% of ALS cases worldwide.
ALS is a progressive neurological disease that attacks nerve cells and causes muscle weakness, paralysis, and ultimately death.
Mutations in the SOD1 gene account for about 2% of all ALS cases. These mutations result in the accumulation of the toxic SOD1 protein, which damages motor neurons.
Mechanism Of Action Of Qalsody
Qalsody tofersen is an antisense oligonucleotide (ASO) designed to bind to and degrade SOD1 mRNA, thereby reducing the production of the toxic SOD1 protein.
By targeting the underlying cause of disease, it has the potential to slow disease progression and improve patients’ quality of life.
Clinical Trial Results
The approval of Tofersen was based on the results of a Phase 3 clinical trial that evaluated the safety and efficacy of Qalsody in ALS patients harboring SOD1 mutations.
Results showed that Qalsody treatment significantly reduced SOD1 protein levels in cerebrospinal fluid compared to placebo. In addition, Qalsody treatment showed a trend toward slowing disease progression and improving patient function.
Patient Access
Tofersen will be available through a restricted program designed to ensure the safe use of the drug and to gather additional data on its long-term safety and effectiveness.
Looking ahead
The approval of Qalsody marks a new era in ALS treatment. Researchers are continuing to study Qalsody’s long-term safety and efficacy, as well as its potential for use in combination with other ALS treatments. In addition, scientists are exploring new therapies for other forms of ALS.
In the future, as more studies are conducted, look for more innovative therapies to benefit a wide range of ALS patients.
However, DengYue’s efforts to increase the affordability of the medicine are critical to ensuring that more patients benefit from it.
We welcome you to reach out to us to get Tofersen price. DengYue Medicine is here to provide detailed medicine information, pricing, and support to ensure a smooth and reliable buying experience.
Feel free to contact us anytime to discuss your needs or ask any questions about the medicine.
Information from HK DengYue, China Drug Import and Export Wholesaler offers this information as a guide, not a substitute for professional medical advice. Consult your doctor before any treatment.



