
Orelabrutinib InnoCare Approval —China’s Original High-Selectivity BTK Inhibitor丨Precision Strike, New Breakthrough
On December 25, 2020, the National Medical Products Administration (NMPA) granted priority review and approval for orelabrutinib tablets (brand name: InnoCare®), a Class 1 innovative drug independently developed by Beijing InnoCare Pharma Technology Co., Ltd.
This milestone marks the official Orelabrutinib InnoCare approval in China.
-1132x800.webp)
This approval represents another major breakthrough in precision therapy for B-cell lymphomas in China, offering a new therapeutic option for patients facing relapse or refractory disease.
Notably, the commercialization and market access of this innovative therapy will be actively supported by DengYue, a leading pharmaceutical wholesale company.
Disease Background and Unmet Clinical Needs
Mantle cell lymphoma (MCL) and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), as important subtypes of B-cell non-Hodgkin lymphoma, present distinct biological features and substantial therapeutic challenges in clinical practice.
Although current standard first-line treatments—including chemotherapy and immunochemotherapy—can induce initial remission in many patients,
durable disease control remains difficult, particularly once relapse or refractory disease develops.
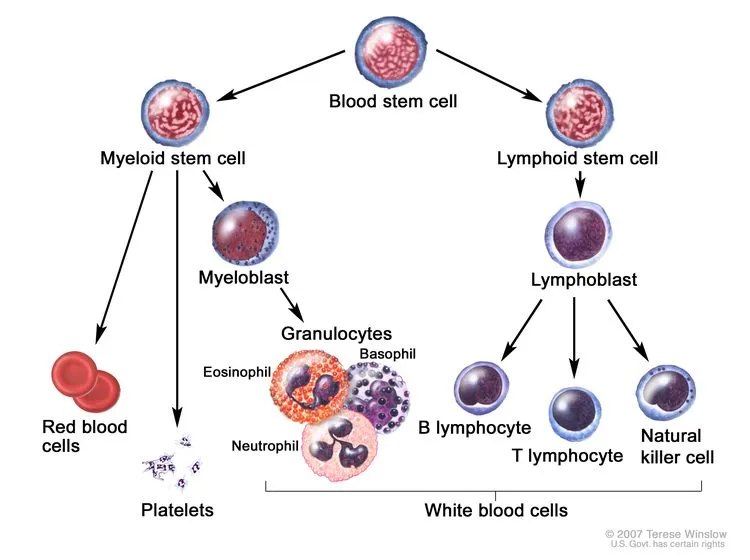
👉 This clinical challenge highlights a critical unmet need for therapies that can more precisely and durably disrupt B-cell survival signaling—most notably the Bruton’s tyrosine kinase (BTK)–driven B-cell receptor pathway, which plays a central role in the pathogenesis and persistence of these diseases.
Targeted Therapy: Progress and Remaining Gaps
In recent years, the advent of targeted therapies has fundamentally reshaped the treatment landscape for B-cell lymphomas.
First-generation Bruton’s tyrosine kinase (BTK) inhibitors, by selectively blocking the B-cell receptor signaling pathway, have provided highly effective, chemotherapy-free treatment options and delivered meaningful survival benefits to patients.
However, clinical experience has also revealed important limitations.
Due to suboptimal target selectivity, these agents may interfere with off-target signaling pathways, resulting in treatment-related adverse events such as:
- Diarrhea,
- Increased bleeding risk
- Atrial fibrillation
These safety concerns can restrict long-term use, negatively affect treatment adherence, and compromise patients’ overall quality of life.
❗ Therefore, how to preserve robust efficacy while further enhancing target selectivity and optimizing safety profiles has become a critical unmet need in clinical practice.
The development of next-generation, highly selective BTK inhibitors represents not only a meaningful breakthrough in overcoming the limitations of existing therapies but also an inevitable path toward achieving more precise, safer, and more durable disease management, reflecting the shared expectations of both clinicians and patients.
In this context, the orelabrutinib InnoCare approval highlights the clinical value of precision-designed BTK inhibition.
Regulatory Basis and Development Journey
🔬 The orelabrutinib InnoCare approval was supported by data from multiple pivotal clinical studies conducted in China.
In a Phase II registrational trial in patients with relapsed or refractory mantle cell lymphoma (MCL), orelabrutinib demonstrated marked antitumor activity.
- As assessed by an independent review committee, the objective response rate (ORR) reached 87.9%, including a complete response (CR) rate of 34.3%.
- Responses were achieved rapidly, and the median duration of response (DOR) showed favorable durability.
✅ In a separate pivotal study involving patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), orelabrutinib likewise delivered strong efficacy alongside a well-tolerated safety profile, with high overall response rates and good treatment tolerability.
Notably, owing to structural optimization and a highly selective design, innocare orelabrutinib exhibited a more favorable safety profile in clinical practice.
Compared with earlier-generation BTK inhibitors, the incidences of common off-target–related adverse events—such as atrial fibrillation, bleeding, and diarrhea—were significantly reduced.
This improved safety margin offers patients a wider therapeutic window and enhances the feasibility of long-term treatment adherence, reflecting the advantages of its refined orelabrutinib mechanism of action.
🟢 Based on these compelling efficacy and safety outcomes, the National Medical Products Administration (NMPA) granted accelerated approval through its Priority Review and Approval pathway.
The orelabrutinib InnoCare approval not only underscores regulatory support for innovative therapies with substantial clinical value but also directly addresses the urgent unmet needs of patients with relapsed or refractory disease.
Dr. Jisong Cui, Co-founder, Chairman, and CEO of InnoCare Pharma (innocare pharma orelabrutinib), commented:
“The clinical data for orelabrutinib are highly encouraging and validate our structure-based approach to designing highly selective molecules.
We will continue to advance its development across broader patient populations and additional indications, staying true to our science-driven and patient-first commitment.”
With this approval, orelabrutinib—marketed under the orelabrutinib brand name InnoCare®—became the 10th original small-molecule kinase inhibitor approved in China and the second domestically developed BTK inhibitor, following zanubrutinib.
This milestone highlights the establishment of a homegrown innovation pipeline in B-cell lymphoma targeted therapy and offers a new treatment option originating from China to patients worldwide.
✨ At the same time, ongoing global development efforts, including exploration in immune-mediated diseases such as orelabrutinib multiple sclerosis and interest surrounding potential orelabrutinib FDA approval, further reflect the expanding international relevance of this molecule.
In-Depth Drug Analysis
BTK is a core regulatory protein in the B-cell receptor (BCR) signaling pathway, functioning as a “master switch” that governs the growth, differentiation, and survival of B cells, including malignant B cells.
🤔 Persistent aberrant activation of this switch drives uncontrolled tumor proliferation and immune evasion.
As a next-generation BTK inhibitor, orelabrutinib binds covalently and with high selectivity to the BTK protein, irreversibly inhibiting its activity.
✨ By precisely “switching off” this critical oncogenic signaling pathway, orelabrutinib blocks the transmission of growth signals and ultimately induces apoptosis of malignant B cells.
-1.webp)
Distinctive Advantage: Structure-Driven Precision Targeting
The differentiated profile of orelabrutinib stems from its carefully engineered molecular structure.
Its core chemical scaffold features a unique monocyclic design, in contrast to the fused-ring structures commonly used in earlier BTK inhibitors.
This innovation enables a more precise fit to the BTK target and substantially reduces off-target interactions with other kinases such as ITK and EGFR.
- High Selectivity Translates into Improved Safety
Preclinical studies have shown that orelabrutinib possesses a markedly higher selectivity index for BTK than first-generation inhibitors.This advantage translates directly into clinical benefit.
In pivotal registrational trials, treatment-related adverse events—particularly those closely associated with off-target effects, including diarrhea, bleeding, and atrial fibrillation—demonstrated a lower incidence and reduced severity, offering patients a more tolerable therapeutic option.
- Irreversible Binding Enables Deep and Durable Inhibition
Through irreversible covalent binding to BTK, orelabrutinib achieves near-complete and sustained target inhibition.
This ensures continued suppression of oncogenic signaling even between dosing intervals, providing a solid pharmacological foundation for deep and durable disease control.
Clinical Value: From “Effective” to “Highly Effective and Patient-Friendly”
The design philosophy behind orelabrutinib reflects the evolution of targeted therapy from broad inhibition to precision-guided intervention.
Beyond delivering strong efficacy—evidenced by high response rates in MCL and CLL/SLL—it achieves a more favorable safety profile through structural optimization.
This combination of being both highly effective and patient-friendly has the potential to help patients achieve long-term, stable disease control without compromising quality of life, fully aligning with the modern, patient-centered approach to cancer treatment.
Conclusion
👉 The approval and launch of orelabrutinib (InnoCare®) represent a landmark achievement in China’s precision oncology landscape.
🏃➡️ More than just a new therapy, orelabrutinib symbolizes the strategic transformation of China’s innovative drug development from “following” to “running alongside,” and in certain areas, even taking the lead.
Through its highly selective design, orelabrutinib has achieved a more optimal balance between clinical efficacy and safety, directly addressing the significant unmet needs of patients with relapsed or refractory B-cell lymphomas.
As one of InnoCare Pharma’s commercialization partners, Dengyue Medicine will leverage its extensive pharmaceutical distribution network and professional service capabilities to facilitate broader clinical access across healthcare institutions, helping more global patients benefit from cutting-edge treatment options.



