
Onradivir Tablets Officially Approved by NMPA丨Marking a Major Breakthrough in Influenza Treatment
Guangdong, China — May 22, 2025 丨 The National Medical Products Administration (NMPA) has officially approved Onradivir Tablets, a Class 1 innovative drug developed by Guangdong Raynovent Biotechnology Co., Ltd. for the treatment of adult patients with uncomplicated influenza A infection.
✨ This approval marks a significant step forward in China’s innovation-driven pharmaceutical development and brings a new, fast-acting, and highly effective treatment option for flu patients.
First-in-Class Antiviral Targeting PB2 Subunit
Onradivir tablets represent the world’s first influenza drug targeting the PB2 subunit of viral RNA polymerase, a crucial component in the virus replication process.
👉 By precisely inhibiting the “cap-binding” function of PB2, onradivir blocks viral RNA transcription and replication at the source—effectively halting the virus from reproducing within host cells.
✅ This groundbreaking mechanism sets Onradivir apart from existing flu medications that only block viral release (such as oseltamivir) or act on other polymerase components (like baloxavir).

Xofluza (Baloxavir Marboxil Tablets) – Influenza | HongKong DengYue Medicine
- Generic Name/Brand Name: Baloxavir Marboxil / Xofluza
- Indications: Influenza in patients aged ≥12 years within 48 hours of symptom onset; post-exposure prophylaxis of influenza in patients aged ≥12 years.
- Dosage Form: Tablet
- Specification: 20mg x 2 tablets
Three Core Advantages: Rapid, Potent, and Low Resistance
1️⃣ Rapid action—Onradivir demonstrated fast symptom relief within 18 hours, with the median time to alleviate seven flu symptoms (TTAS) shortened by 24.52 hours compared to placebo.
2️⃣ Potent antiviral efficacy—Laboratory studies show Onradivir’s antiviral activity is 50× stronger than baloxavir and over 1,000× stronger than oseltamivir, with an EC₉₀ of 0.05 nM.
It rapidly reduces viral load within 24 hours and significantly shortens the time to viral negativity.
3️⃣ Low resistance potential—Onradivir maintains strong inhibitory activity against oseltamivir-resistant, baloxavir-resistant, and highly pathogenic avian influenza strains, showing a lower mutation rate than existing antivirals.
Strengthening China’s Influenza Defense
🌟 The approval of Onradivir not only enriches China’s influenza drug arsenal but also highlights a significant leap in domestic antiviral innovation.
😷 As influenza and COVID-19 continue to co-circulate, Raynovent Biotechnology is implementing its “COVID-Influenza Integrated Prevention and Control Program,” promoting synergy in respiratory disease management.
Raynovent’s COVID-19 drug, Leritavir Tablets, has been widely adopted in clinical use and recently received its first prescription at Macau United Medical Center.
Onradivir Tablets From Laboratory to Patients
From NMPA approval to its first retail launch in Guangzhou, Anruiwei is rapidly transitioning from the laboratory to patients’ hands.
🙂 As a homegrown antiviral drug with independent intellectual property rights, Onradivir tablets reinforce China’s capacity for pharmaceutical innovation and contribute to national public health resilience.
💁♂️ With the approval and nationwide launch of Onradivir Tablets, DengYue Medicine—a Chinese pharmaceutical distributor—will continue to follow the development of innovative antiviral therapies.
🌐 Leveraging its global distribution network, Dengyue is committed to introducing more high-quality Chinese innovative drugs to international markets, offering safe and reliable treatment options for patients worldwide.
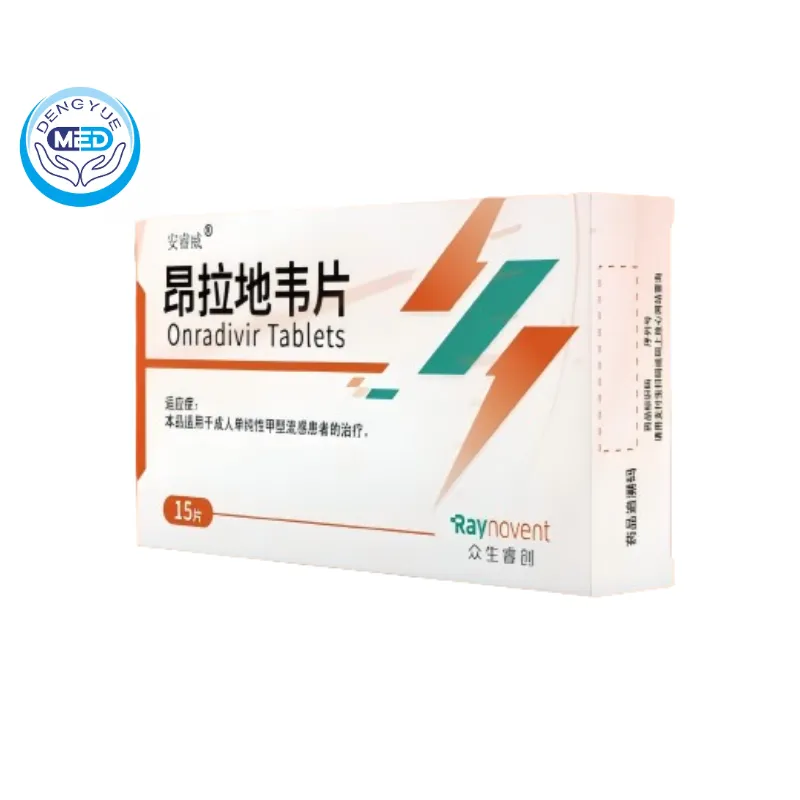
Raynovent (Onradivir) – Influenza A | HongKong DengYue Medicine
- Generic Name/Brand Name: Onradivir/An Rui Wei
- Indications:Adult uncomplicated influenza A treatment.
- Dosage Form: Tablets
- Specification: 200 mg × 15



