
A Breakthrough in Fatal Pulmonary Fibrosis! World’s First PDE4B Inhibitor—Nerandomilast Approved in China
For approximately 5.6 million patients worldwide living with progressive pulmonary fibrosis (PPF), every breath is accompanied by the relentless and irreversible scarring of their lungs.
This is a fatal pulmonary disease, with nearly half of patients dying within five years of diagnosis, while facing an average diagnostic delay of up to two years and a treatment gap affecting close to half of those diagnosed.
As reported by DengYue Medicine, this dire landscape has now welcomed its most significant breakthrough in a decade.
Boehringer Ingelheim announced that its global first-in-class oral phosphodiesterase 4B (PDE4B) inhibitor, Nerandomilast (brand name: Boyoowei®), has officially received marketing approval from the National Medical Products Administration (NMPA) of China.
✨ Nerandomilast Approved in China, it not only marks the first new pulmonary fibrosis drug in ten years to achieve primary endpoints in pivotal Phase III clinical trials but also accomplishes “global synchronization,” receiving approval in China just two weeks after its U.S. authorization.
This milestone signifies China’s full alignment with the international forefront in innovative treatments for respiratory fibrotic diseases.
Advancing Disease Understanding: From IPF to a Broader Disease Spectrum
To comprehend the significance of this approval, we must first examine the most typical form of pulmonary fibrosis—Idiopathic Pulmonary Fibrosis (IPF).
IPF is a chronic, progressive fibrosing interstitial pneumonia of unknown cause, pathologically characterized by “honeycombing” changes in the lungs and an irreversible decline in pulmonary function.
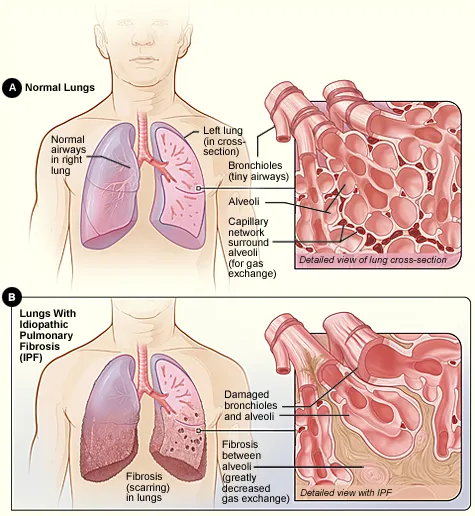
The median survival after diagnosis is only 3–5 years, with a mortality rate higher than that of most cancers.
Over the past decade, only two drugs (nintedanib and pirfenidone) have been proven to slow disease progression in IPF, yet clinical needs remain far from being met.
The indication for which Nerandomilast is now approved—Progressive Pulmonary Fibrosis (PPF)—represents a broader clinical concept.
It encompasses not only IPF but also pulmonary fibrosis resulting from various known causes, such as autoimmune diseases (e.g., rheumatoid arthritis, scleroderma) and environmental exposures, which similarly exhibit persistent worsening features.
👉 This patient population accounts for approximately 18% to 32% of all interstitial lung disease cases.
Despite sharing a disease progression pattern and high mortality rate similar to IPF, these patients have long lacked a dedicated therapy validated by large-scale Phase III clinical trials.
Breakthrough Mechanism: Targeting the Core of Fibrosis and Inflammation
Nerandomilast is a small-molecule drug with “first-in-class” status.
🎯 It exerts triple effects—immune modulation, vascular protection, and anti-fibrotic action—by highly selectively inhibiting the PDE4B isoenzyme, which is highly expressed in the lungs.

This novel mechanism operates through a pathway entirely different from existing therapies, providing a new scientific weapon in the fight against the complex disease of pulmonary fibrosis.
“The progression of pulmonary fibrosis is closely linked to inflammation and abnormal repair,” stated a respiratory disease expert involved in the clinical trials.
“Nerandomilast selectively targets PDE4B, regulating multiple pathogenic pathways upstream. Its innovative design represents the new direction of precision medicine.”
Solid Data Support
The approval is based on the positive results of the pivotal global Phase III clinical trial named FIBRONEER-ILD™. This study is the first successful Phase III trial in this field in a decade and the largest clinical project of its kind to date.
The data show that nerandomilast significantly slows the deterioration of patients’ lung function.
- At week 52 of treatment, the decline in forced vital capacity (FVC)—a core measure of lung function—was as high as -165.8 mL in the placebo group.
- In contrast, FVC decline in the Nerandomilast 9 mg group was only -84.6 mL, and -98.6 mL in the 18 mg group.
This means Nerandomilast reduced the rate of lung function decline by approximately 49% to 40% compared to placebo.
✅ Even more encouragingly, the study data also indicated a nominally significant reduction in mortality risk in the Nerandomilast treatment group, offering hope for extended survival in this fatal disease.
1️⃣ Regarding safety, Nerandomilast demonstrated good tolerability, with overall discontinuation rates similar to the placebo group.
2️⃣ The most common adverse reaction was dose-dependent diarrhea, which was manageable in most cases.
3️⃣ Notably, routine liver function monitoring is not required during treatment, greatly simplifying patient management and improving treatment convenience.
Looking ahead, with its proven efficacy and favorable safety profile, Nerandomilast is poised to transform the current treatment paradigm—ushering in a new era of “triple therapy” and moving beyond the longstanding “two-pillar” approach.
Looking Ahead: Expanding Indications to Benefit a Broader Patient Population
The launch of Nerandomilast (Boyoowei®) represents more than just the introduction of a new drug—it marks a renewed offensive against a fatal disease.
Supported by robust Phase III clinical data, it brings new hope to approximately 5.6 million patients and their families struggling with respiratory challenges, offering the promise of delaying disease progression and improving quality of life.
This milestone heralds a new chapter in the treatment of pulmonary fibrosis in China.
👉 With the successful launch of Nerandomilast (Boyoowei®) in China, as a global pharma partner, we are accelerating the establishment of a nationwide drug accessibility network to ensure this breakthrough therapy can quickly reach a wide range of clinical patients.




