
Lisaftoclax Tablets: The First Domestic Bcl-2 Inhibitor Approved for CLL/SLL Treatment in China
Suzhou Ascentage Pharma has achieved a significant milestone with the conditional approval of Lisaftoclax Tablets (brand name: Lishengtuo, research code: APG-2575) by the National Medical Products Administration (NMPA).
✨ This approval is for the treatment of chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) in adult patients who have failed at least one prior systemic therapy, including a Bruton’s tyrosine kinase (BTK) inhibitor.
👉 This marks Lisaftoclax as the first Bcl-2 inhibitor approved for CLL/SLL in China and the second globally.
Innovative Mechanism of Action of Lisaftoclax Tablets
Lisaftoclax is a novel oral Bcl-2 selective inhibitor that works by selectively inhibiting the Bcl-2 protein, restoring the normal apoptotic process in cancer cells, thereby targeting and eliminating tumor cells.
🔬 The Bcl-2 protein is an important anti-apoptotic factor that is overexpressed in many hematologic malignancies, particularly in CLL/SLL, allowing tumor cells to evade apoptosis.
As a result, Bcl-2 inhibitors offer an innovative treatment option for CLL/SLL and other blood cancers.
Clinical Data Supporting the Efficacy of Lisaftoclax
The approval of Lisaftoclax is based on the results of a key Phase II clinical study (APG2575CC201) conducted in China.
👉 This study evaluated the efficacy and safety of Lisaftoclax as a single-agent treatment for relapsed/refractory (R/R) CLL/SLL patients.
✅ The results showed that for patients who failed prior treatment with BTK inhibitors and/or immunochemotherapy, lisaftoclax demonstrated significant efficacy, achieving the preset study endpoint with an Overall Response Rate (ORR), and showed good safety.
Global Phase III Clinical Trials Progress
🌟 Currently, Lisaftoclax has entered four global Phase III clinical trials, covering several indications, including CLL/SLL, acute myeloid leukemia (AML), and myelodysplastic syndromes (MDS).
💁♂️ One of these studies has been granted FDA approval, further expanding the global clinical reach of Lisaftoclax.
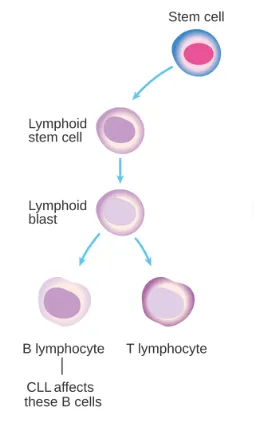
CLL/SLL: A Disease in Need of New Therapies
CLL/SLL is a mature B-cell clonal proliferative tumor that primarily affects elderly patients.
It is the most common form of adult leukemia in Western countries. Each year, more than 100,000 new cases are diagnosed worldwide.
⚠️ Although immunochemotherapy and BTK inhibitors have significantly improved the initial remission rates for CLL/SLL patients, existing treatments still face major challenges.
These include limited depth of response, a high risk of relapse, and long-term toxicities.
❗ For relapsed or refractory (R/R) CLL/SLL patients, the need for safer and more effective treatment options remains urgent.
Bcl-2 Inhibitors: A Revolutionary Breakthrough for CLL/SLL Treatment
Bcl-2 inhibitors work by blocking the function of the Bcl-2 protein and restoring the natural process of apoptosis in cancer cells.
This mechanism helps increase tumor cell death and improve treatment outcomes.
The approval of Lisaftoclax marks a major advance in the treatment of CLL/SLL and other blood cancers.
Lisaftoclax tablets are the first Bcl-2 inhibitor approved in China for CLL/SLL, providing a new treatment option for patients.
Conclusion: New Hope for CLL/SLL Patients
🌐 The approval of Lisaftoclax Tablets not only fills a critical gap in the treatment of CLL/SLL but also brings new hope to patients.
As the second Bcl-2 inhibitor approved globally, Lisaftoclax shows significant potential
⌛ As more innovative drugs like Lisaftoclax Tablets receive market approval, China’s pharmaceutical innovation is entering a new stage of accelerated growth.
As a professional pharmaceutical distributor, Hong Kong DengYue Medicine remains committed to closely following the approval and commercialization of innovative drugs, providing efficient and compliant distribution support to ensure high-quality medicines reach more patients in need.



