Gomekli (Mirdametinib): Hope for Inoperable NF1 Cases
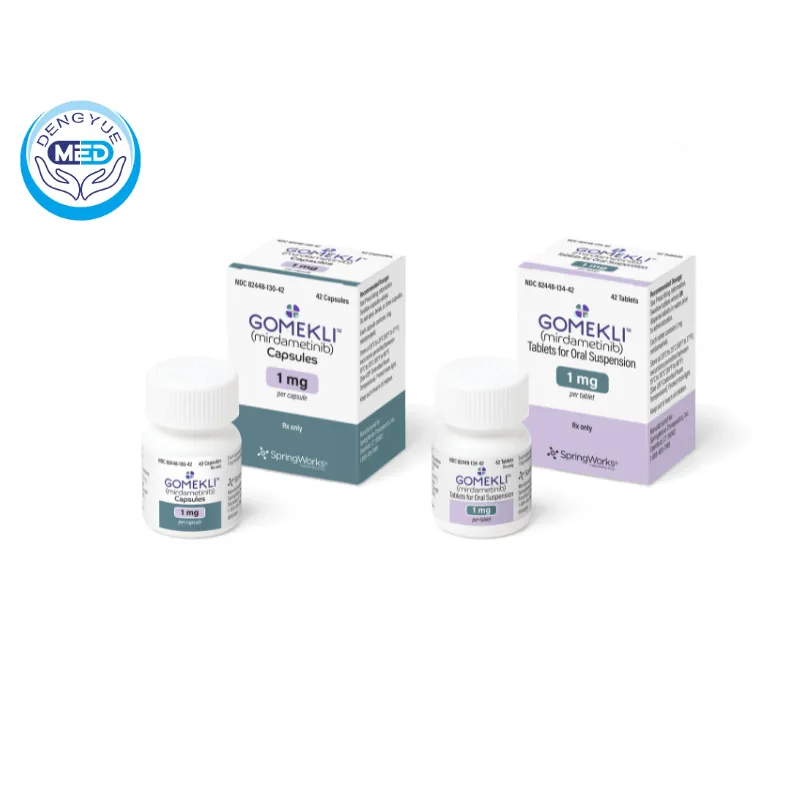
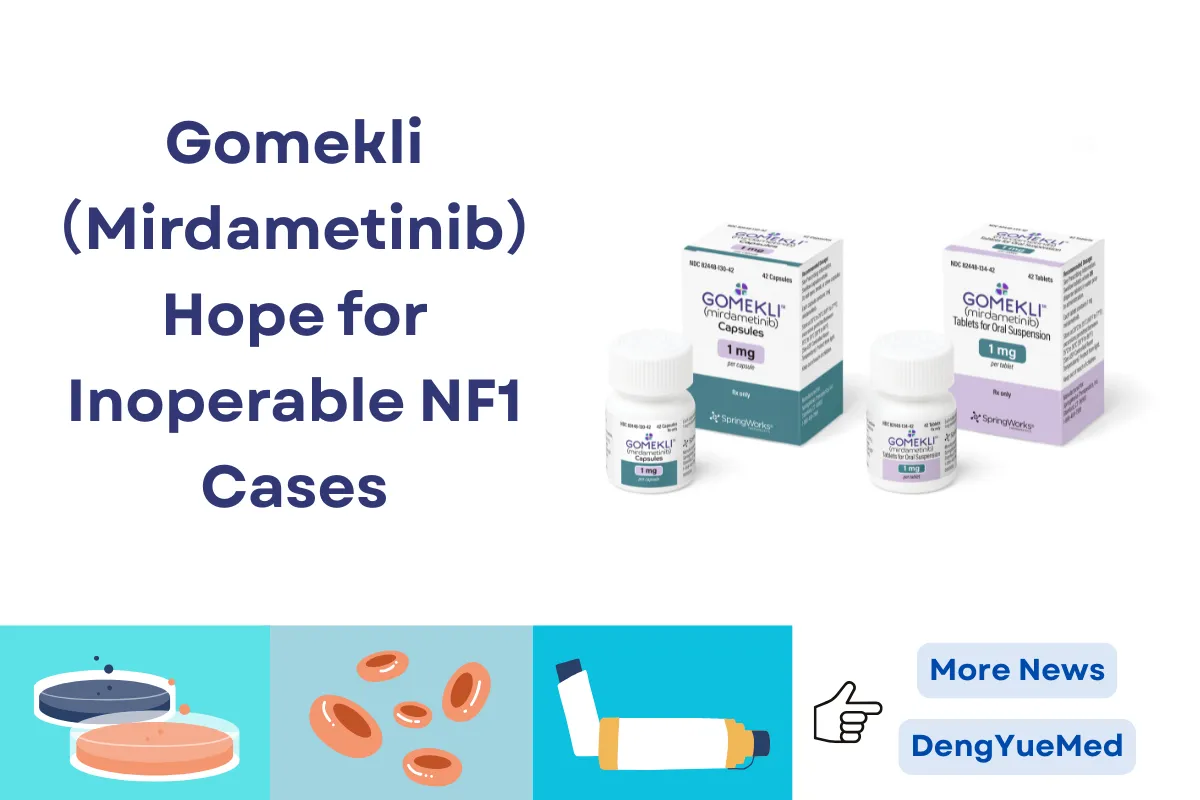
Gomekli™ (mirdametinib) has emerged as a significant advancement in the treatment of neurofibromatosis type 1 (NF1) with symptomatic plexiform neurofibromas (PN), offering new hope to patients aged 2 years and older.
🧬 Understanding Neurofibromatosis Type 1 (NF1) and Plexiform Neurofibromas
NF1 is a genetic disorder characterized by the development of tumors along nerves in the skin, brain, and other parts of the body.
Plexiform neurofibromas are complex nerve sheath tumors associated with NF1 that can cause significant morbidity due to their size, location, and potential for malignant transformation.
NF1, affecting 1 in 3,000 individuals worldwide, is caused by mutations in the NF1 gene, leading to uncontrolled RAS/MAPK pathway activation.
Plexiform neurofibromas—present in 30–50% of NF1 patients—are complex, slow-growing tumors that infiltrate nerves, causing pain, disfigurement, functional impairments, and risk of malignant transformation to malignant peripheral nerve sheath tumors (MPNSTs).
💊 Mirdametinib: The First FDA-Approved Treatment for NF1-PN
On February 11, 2025, the U.S. Food and Drug Administration (FDA) approved Gomekli™ (mirdametinib) for treating adult and pediatric patients aged 2 years and older with NF1 who have symptomatic PN not amenable to complete surgical resection.
This approval was based on the Phase 2b ReNeu trial results, which demonstrated significant tumor reduction and symptomatic improvement in patients.
🔬 Mechanism of Action: Targeting the MEK Pathway
Mirdametinib is a selective MEK1/2 inhibitor that targets the mitogen-activated protein kinase (MAPK) pathway, which is often dysregulated in NF1-associated tumors.
By inhibiting this pathway, Gomekli™ helps reduce tumor growth and associated symptoms.
📊 Clinical Efficacy: Results from the ReNeu Trial
The Phase 2b ReNeu trial enrolled 114 patients with NF1-PN aged 2 years and older. The study reported an objective response rate (ORR) of 41% in adults and 52% in pediatric patients, indicating a significant reduction in tumor size.
Furthermore, 88% of adults and 90% of children with a confirmed response maintained their response for at least 12 months, and approximately half sustained it for 24 months.
As a selective MEK1/2 inhibitor, Gomekli™ disrupts hyperactive MAPK signaling in NF1-deficient Schwann cells, uniquely addressing the biologic driver of PN growth while sparing healthy tissues.
⚠️ Safety Profile and Adverse Reactions
Common adverse reactions observed in the ReNeu trial included rash, diarrhea, nausea, vomiting, and fatigue. Serious adverse events were infrequent but included ocular toxicity and cardiac dysfunction.
Regular monitoring is recommended during treatment.
🏥 Administration and Dosage
Gomekli™ is available in capsule form and as tablets for oral suspension, allowing for flexible dosing in both adults and children.
Dosage is determined based on body surface area and administered twice daily in 28-day cycles. While generally well-tolerated, clinicians monitor for:
- GI symptoms (rash [45%], diarrhea [32%], managed with supportive care)
- Ocular toxicity (retinal vein occlusion, blurred vision)
- Cardiac effects (asymptomatic EF decline in 8% of trial participants)
🔗 Conclusion
The approval of Gomekli™ drug marks a significant milestone in managing NF1-associated plexiform neurofibromas, providing an effective treatment option for patients who previously had limited therapeutic choices.
With ongoing research and commitment from companies like Dengyue, the future holds promise for those living with NF1.
About DengYueMed – HK Drug Wholesale Distributor
As a legally compliant drug import and export company, DengYueMed is certified by the pharmacy & poisons board of Hong Kong — you can verify our qualification on their official website.

Our efforts to improve the affordability of Gomekli medicine aim to ensure that more patients can benefit from this important medication.
HK DengYue provides detailed medicine information, transparent pricing, and responsive support to ensure a smooth and reliable buying experience.
Feel free to reach out anytime to discuss your needs or ask questions about the medicine.
We welcome you to contact us for a consultation.



