
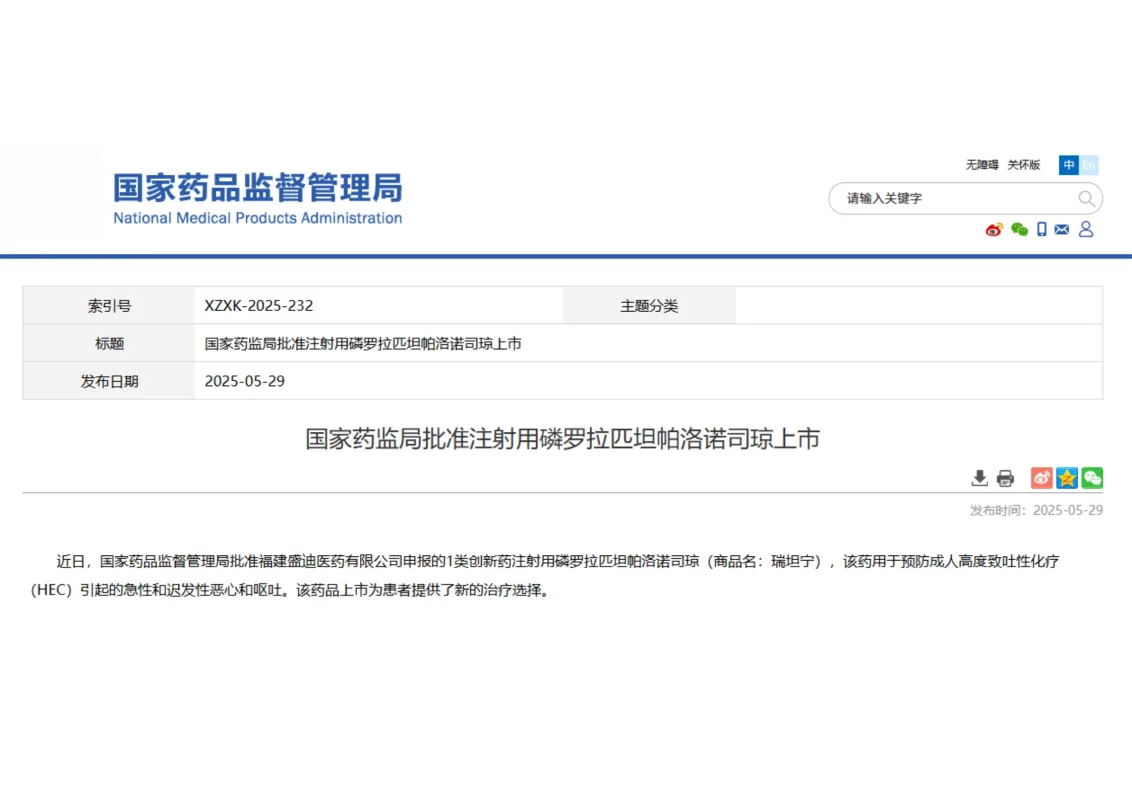
Fosrolapitant and Palonosetron Hydrochloride Injection Receives Approval丨Offering New Hope for Chemotherapy-Induced Nausea and Vomiting Management
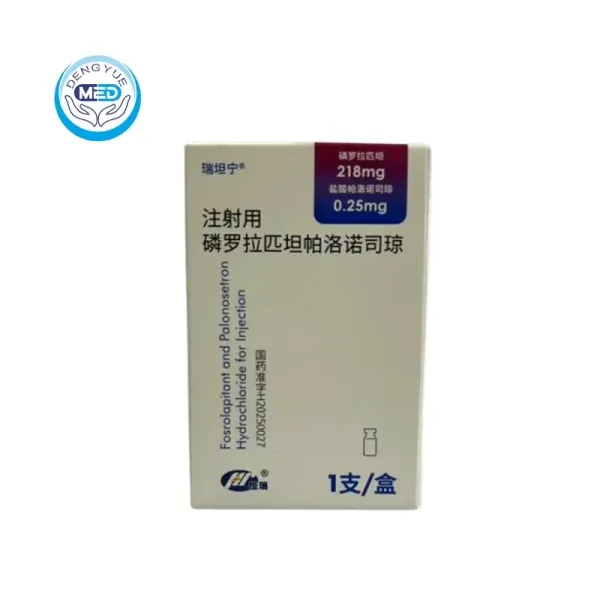
Recently, Fujian Shengdian Pharmaceutical Co., Ltd., a subsidiary of Hengrui Medicine, received approval from the National Medical Products Administration (NMPA) for the launch of Fosrolapitant and Palonosetron Hydrochloride Injection.
💊 This novel Class 1 innovative drug is indicated for the prevention of acute and delayed nausea and vomiting induced by highly emetogenic chemotherapy (HEC) in adult patients.
Notably, it is China’s first long-acting, original combination antiemetic injection, marking a significant step forward in improving chemotherapy-related quality of life for cancer patients.
Innovative Mechanism and Clinical Benefits
💉 Fosrolapitant and Palonosetron Hydrochloride Injection combines a novel long-acting NK-1 receptor antagonist (fosrolapitant) with the second-generation 5-HT3 receptor antagonist (palonosetron), targeting both central and peripheral pathways of the vomiting reflex.
✅ Thanks to its nearly 8-day half-life, a single injection per chemotherapy cycle can cover the entire risk period, including acute (0–24 hours), delayed, and extended delayed (120–168 hours) phases of chemotherapy-induced nausea and vomiting (CINV).
The approval is supported by the pivotal Phase III PROFIT study (NCT05509634), published in the renowned Journal of Clinical Oncology (JCO).
🤔 The study demonstrated that fosrolapitant and palonosetron combined with dexamethasone achieved over 90% complete response rates in both acute and extended delayed phases, while maintaining a favorable safety profile.
This once-per-cycle regimen offers a convenient and effective solution, improving patient adherence and supporting the standardized management of CINV in clinical practice.
Addressing a Critical Unmet Need
CINV remains one of the most common adverse effects of chemotherapy, significantly affecting patient quality of life and treatment adherence.
For HEC regimens, acute nausea and vomiting rates can exceed 90%, while delayed symptoms occur in more than 50% of patients.
🔬 Studies indicate that approximately 32% of patients may interrupt or discontinue chemotherapy due to CINV, underscoring the urgent need for effective preventive strategies.
Traditional antiemetic regimens often require multiple doses due to shorter half-lives, complicating adherence and full coverage of the risk period.
🙂 Fosrolapitant and Palonosetron Hydrochloride Injection address these challenges by providing a long-acting, dual-targeted antiemetic approach, simplifying therapy while maintaining robust efficacy across the entire chemotherapy cycle.
Expert Commentary about Fosrolapitant and Palonosetron Hydrochloride Injection
Professor Liang Jun, Director of the Oncology Center at Peking University International Hospital, emphasized the clinical significance of this innovative treatment.
He noted that the dual-target mechanism blocks both central and peripheral pathways of nausea and vomiting, ensuring comprehensive protection.
With its long-acting profile and single-dose convenience, this therapy reduces patient burden, minimizes interruptions in chemotherapy, and enhances treatment outcomes.
Looking Ahead
As an international innovative pharmaceutical company, Hengrui Medicine continues to advance research and development in areas of high clinical need.
With over 23 Class 1 innovative drugs already approved in China, including numerous oncology therapies, the launch of fosrolapitant and palonosetron injection reinforces the company’s commitment to improving patient survival and quality of life.
Its introduction paves the way for full-cycle, standardized, and individualized CINV management, representing a new chapter in supportive cancer care in China.
💁♂️ DengYue Medicine will continue to monitor advancements in this field and is committed to providing patients and healthcare professionals with up-to-date drug information and support services.
👉 By the way, this tip can help you to verify the authenticity of Chinese medicines when you use the drug.



