
7 Key Insights About Duvyzat: A New Hope for Duchenne Muscular Dystrophy
Table of Contents
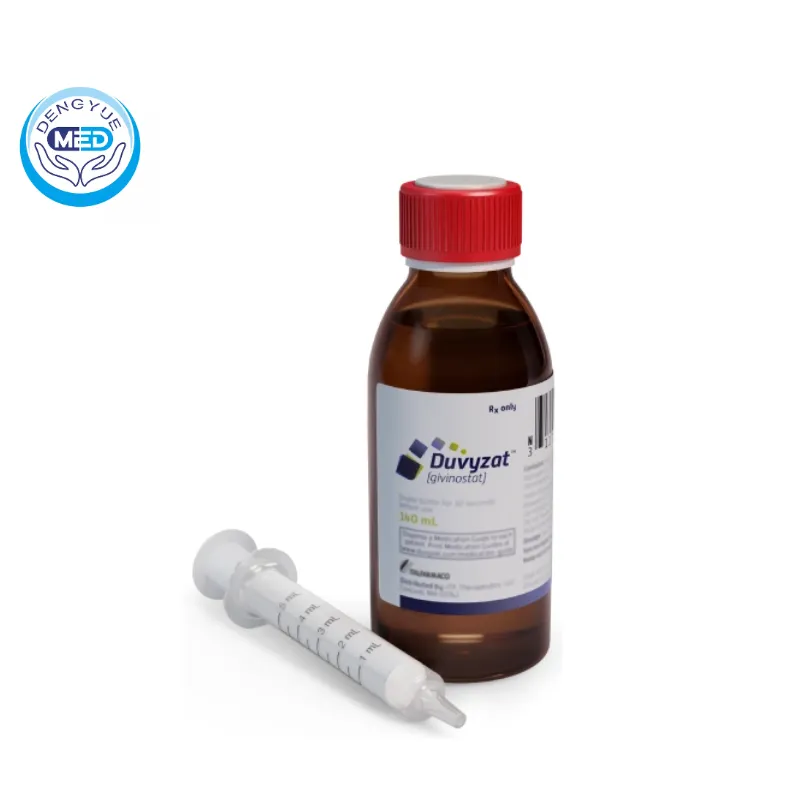
Introduction
Duchenne Muscular Dystrophy (DMD) is a severe genetic disorder characterized by progressive muscle degeneration and weakness.
In March 2024, the U.S. Food and Drug Administration (FDA) approved Duvyzat (givinostat), marking a significant advancement in DMD treatment.
This blog explores 7 essential insights about Duvyzat, shedding light on its mechanism, clinical efficacy, and impact on the DMD community.
What Is The Drug Duvyzat Used For?
First Nonsteroidal Treatment Approved for All DMD Genetic Variants
Duvyzat stands out as the first nonsteroidal medication approved by the FDA to treat DMD across all genetic variants in patients aged six and older.
Unlike previous treatments targeting specific mutations, it offers a mutation-agnostic approach, broadening therapeutic options for a wider patient population.
Duchenne muscular dystrophy (DMD)
The most common form of muscular dystrophy in childhood and usually affects males.
It is a rare neurological disorder that results in progressive muscle weakness due to a lack of a muscle protein called antimyotrophin.
Over time, the muscles deteriorate, leading to reduced walking and muscle strength, and eventually breathing difficulties and ultimately premature death.
Mechanism Of Action
Targets HDAC Overactivity to Protect Muscle Function
Duvyzat is a histone deacetylase (HDAC) inhibitor that addresses the overactivity of HDAC enzymes in dystrophic muscle tissue—a hallmark of DMD pathology.
By inhibiting HDACs, Givinostat aims to reduce inflammation, decrease fibrosis, and promote muscle fiber regeneration, thereby preserving muscle function
Efficacy In Slowing Disease Progression
The only nonsteroidal treatment indicated for Duchenne muscular dystrophy in people 6 years of age and older, regardless of their genetic variant or ability to walk.
Clinical trials have shown that Givinostat can significantly slow the progression of DMD.
In a pivotal Phase 3 study, patients treated with Duvyzat exhibited a ~40% slower decline in functional abilities over 18 months, as measured by the four-stair climb test, compared to those on placebo.
Delays Disease Progression by 2 Years
A simulation model using data from the Duchenne Regulatory Science Consortium indicated that Duvyzat could delay DMD progression by approximately two years compared to standard care.
This delay translates to extended mobility and improved quality of life for patients.
Oral Administration Enhances Patient Compliance
Duvyzat is administered orally, offering a more convenient alternative to injectable treatments.
This ease of administration can lead to better adherence to the treatment regimen, especially important for pediatric patients and their caregivers.
Common Side Effects Are Generally Manageable
The most frequently reported side effects of Duvyzat include diarrhea, abdominal pain, thrombocytopenia (low platelet count), nausea/vomiting, elevated triglyceride levels, and fever.
These adverse effects are typically manageable with appropriate medical supervision.
How Much Does Duvyzat Cost?
The cost of Duvyzat (givinostat) varies based on factors such as dosage and pharmacy pricing.
Here’s an overview of its pricing:
💰 Retail Pricing
Annual Cost: The average annual cost of Duvyzat is approximately $700,000, based on dosing for a typical patient.
Per Bottle Cost: For a 140 mL bottle of 8.86 mg/mL oral suspension, prices range from $38,149.10 to $40,890.68, depending on the pharmacy.
For personalized cost information, please feel free to consult with DengYue Medicine for more bulk pricing.
Conclusion
Duvyzat represents a promising advancement in the treatment of Duchenne Muscular Dystrophy, offering a novel, nonsteroidal, and mutation-agnostic therapy.
Its ability to slow disease progression and improve patient outcomes marks a significant step forward in DMD care.
As research continues, Duvyzat may pave the way for further innovations in managing this challenging condition.
This product is a prescription-only drug, meaning it can only be purchased (or sold) with a valid prescription.
Specifically, DUVYZAT is a drug used to treat Duchenne muscular dystrophy (DMD) in individuals aged 6 years or older and was approved by the FDA in March 2024 based on the positive results of the phase 3 EPIDYS clinical trial (NCT02851797 – see https://www.fda.gov/news-events/press-announcements/fda-approves-nonsteroidal-treatment-duchenne-muscular-dystrophy
About DengYueMed – HK Drug Wholesale Distributor
As a legally compliant drug import and export company, DengYueMed is certified by the pharmacy & poisons board of Hong Kong — you can verify our qualification on their official website.

Our efforts to improve the affordability of Duvyzat medicine aim to ensure that more patients can benefit from this important medication.
HK DengYue provides detailed medicine information, transparent pricing, and responsive support to ensure a smooth and reliable buying experience.
Feel free to reach out anytime to discuss your needs or ask questions about the medicine.
We welcome you to contact us for a consultation.



