
New Hope for Advanced Nasopharyngeal Carcinoma: Becotatug Vedotin Receives Regulatory Approval
Hong Kong DengYue Medicine News – October 30, 2025 – Lepu Biopharma Co., Ltd. (“Lepu Bio”) announced that its self-developed innovative antibody-drug conjugate (ADC)—Becotatug Vedotin for Injection (trade name: Meiyouheng®)—has been formally approved for marketing by the National Medical Products Administration (NMPA) of China.
Becotatug Vedotin Receives Regulatory Approval for the treatment of adult patients with recurrent or metastatic nasopharyngeal carcinoma (R/M NPC) who have previously failed at least two lines of systemic chemotherapy and PD-1/PD-L1 inhibitor therapy.
It stands as China’s first and a globally leading EGFR-targeted ADC, bringing a new precision treatment option to patients facing the challenge of later-line therapy.

Addressing Unmet Clinical Needs in Late-Line Treatment
Nasopharyngeal carcinoma (NPC) is a malignancy with high incidence in China, particularly in southern regions, exhibiting distinct epidemiological characteristics.
Approximately 70% of global cases are concentrated in East and Southeast Asia, with China accounting for nearly half of the world’s newly diagnosed cases.

Currently, platinum-based chemotherapy combined with PD-1/PD-L1 inhibitors has become the first-line standard treatment for recurrent or metastatic nasopharyngeal carcinoma, significantly improving survival outcomes for some patients.
🤔 However, clinical data indicate that over half of the patients still experience disease progression after initial therapy, facing a severe challenge of limited subsequent treatment options.
For patients who have failed two or more lines of systemic chemotherapy and immunotherapy, traditional treatment approaches show limited efficacy.
Existing late-line chemotherapy regimens often yield objective response rates below 15%, with median progression-free survival typically less than three months.
This results in poor quality of life for patients and highlights a significant unmet clinical need.
This treatment dilemma not only affects patients’ survival prospects but also imposes a heavy burden on families and society.
✨ Against this backdrop, the Becotatug Vedotin receives regulatory approval holds important clinical significance.
Targeting the highly expressed EGFR in nasopharyngeal carcinoma (approximately 89% of advanced cases show high EGFR expression), this drug achieves precision treatment through innovative ADC technology.
It provides a new therapeutic option for patients with advanced nasopharyngeal carcinoma who have failed multiple prior lines of treatment, offering the potential to improve survival outcomes and quality of life for this patient population.
Innovative ADC Mechanism: “Biological Missile” Precision Targeting Opens New Paradigm in Targeted Therapy
The antibody-drug conjugate (ADC) technology employed by Becotatug Vedotin represents a cutting-edge advancement in the field of tumor-targeted therapy.
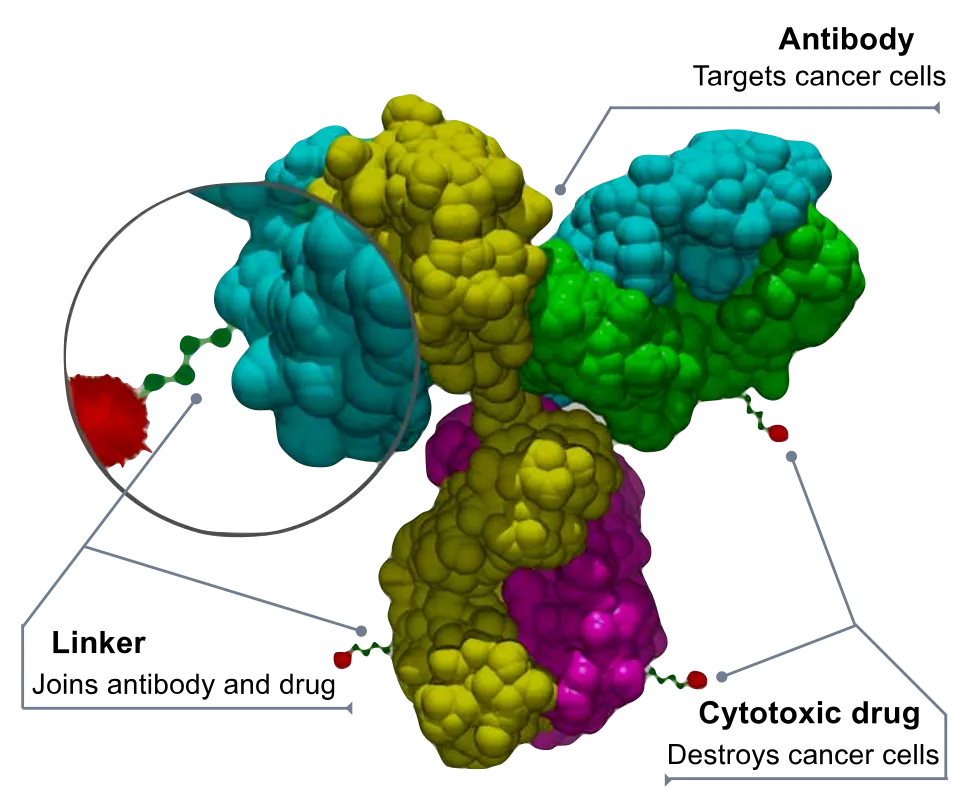
Its mechanism of action can be likened to a “biological missile” equipped with an intelligent guidance system, achieving precise elimination of tumor cells through the sophisticated coordination of three core components:
- Intelligent Guidance System (Targeting Antibody): The engineered EGFR monoclonal antibody carried by the drug functions as the missile’s guidance system, capable of accurately recognizing and binding with high affinity to the overexpressed EGFR protein on the surface of tumor cells.
✅ This characteristic allows it to effectively distinguish between tumor cells and normal tissues within the complex human environment, laying the foundation for precise delivery.
- Controlled Release Mechanism (Cleavable Linker): The valine-citrulline linker connecting the antibody to the cytotoxic drug is ingeniously designed to remain highly stable in the bloodstream, minimizing “off-target toxicity.”
✅ It is specifically cleaved only after entering the tumor cell by proteases unique to the lysosome, enabling a “tumor microenvironment-responsive” drug release that significantly enhances treatment selectivity.
- High-Efficiency Payload (Cytotoxic Agent): The monomethyl auristatin E (MMAE) carried by the drug is a validated potent microtubulin inhibitor, with efficacy hundreds of times greater than traditional chemotherapy agents.
✅ This “high-potency payload” strategy allows ADC drugs to achieve significant anti-tumor effects at very low doses while limiting their exposure to normal cells throughout the body through the precise delivery system.
✨ Unlike the “indiscriminate killing” of traditional chemotherapy or the “signal-blocking” effect of conventional targeted therapies, the ADC mechanism of Becotatug Vedotin enables a cascade process of “precise recognition—efficient internalization—targeted destruction.”
This design not only markedly enhances cytotoxic efficacy against tumor cells with high EGFR expression but also, by reducing systemic drug exposure, may improve the safety profile commonly associated with traditional chemotherapy.
This technological innovation ultimately translates into substantial improvements in the patient treatment experience—shifting the treatment paradigm for advanced nasopharyngeal carcinoma from the traditional “fighting poison with poison” approach to “precision-guided targeting.”
For patients, this means achieving stronger therapeutic efficacy while potentially enduring less treatment-related suffering, thereby truly enhancing quality of life and dignity on the basis of extended survival.
Outstanding Efficacy Data with Significant Survival Benefits
The approval of Becotatug Vedotin is based on a pivotal phase III registrational clinical study presented as a “Late-Breaking Abstract” at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting, one of the world’s premier oncology conferences.
The study enrolled 173 patients with advanced nasopharyngeal carcinoma who had failed multiple prior lines of therapy, directly comparing the efficacy and safety of Becotatug Vedotin versus standard chemotherapy (capecitabine or docetaxel).
The study data demonstrate clear advantages:
- Significantly Improved Tumor Response Rates: As assessed by an independent review committee, the objective response rate (ORR) in the Becotatug Vedotin arm reached 30.2%, nearly three times higher than the chemotherapy arm’s 11.5%, indicating tumor shrinkage in a greater proportion of patients.
- Markedly Prolonged Disease Control Duration: The median progression-free survival (PFS) for patients in the Becotatug Vedotin arm was 5.8 months, significantly superior to the 2.8 months in the chemotherapy arm, representing a 37% reduction in the risk of disease progression or death.
- Extended Overall Survival: An interim analysis revealed that the median overall survival (OS) in the Becotatug Vedotin arm was 17.1 months, compared to 12.0 months in the chemotherapy arm, providing patients with a clear and clinically meaningful survival benefit.
- Consistent Benefit Across Key Subgroups: Further analysis confirmed that the survival benefits were consistently observed across various predefined patient subgroups, including those with liver metastases and patients previously treated with multiple lines of therapy, underscoring the robustness of the treatment effect.
- Manageable Safety Profile Supporting Clinical Utility: The safety profile of Becotatug Vedotin was manageable and consistent with the expected effects of an ADC targeting EGFR, with no new safety signals identified.
Collectively, these robust clinical trial results demonstrate that Becotatug Vedotin provides statistically significant and clinically meaningful improvements across all key efficacy endpoints—ORR, PFS, and OS—compared to standard chemotherapy in heavily pretreated advanced nasopharyngeal carcinoma patients.
Accelerating Access, A Promising Future
The National Medical Products Administration (NMPA) granted approval for this product through the “Priority Review and Approval Procedure” with “conditional approval,” demonstrating strong support for domestically developed innovative drugs with outstanding clinical value.
This approval marks a significant milestone for Lepu Bio in the field of oncology treatment innovation.
Industry experts commented, “Becotatug Vedotin receiving regulatory approval signifies that China’s nasopharyngeal carcinoma treatment has officially entered the era of ADC precision therapy.
It provides patients who have failed existing standard therapies with a powerful and effective weapon, and its clear survival benefits are encouraging.”
Lepu Bio stated that it will fully advance the commercialization process of this drug to ensure its benefits reach patients nationwide as soon as possible.

Concurrently, the company is actively conducting clinical research on the drug for other EGFR-high-expressing solid tumors (such as head and neck squamous cell carcinoma, non-small cell lung cancer, etc.), aiming to expand its therapeutic potential and benefit a broader patient population.
👉 If you would like to learn more about the latest medical insights on nasopharyngeal carcinoma, DengYue invites you to read our article, “From a Regional Mystery to a Global Breakthrough: The Scientific Journey of Nasopharyngeal Carcinoma Treatment.”



