Baqsimi: A Revolutionary Breakthrough In Diabetes Treatment
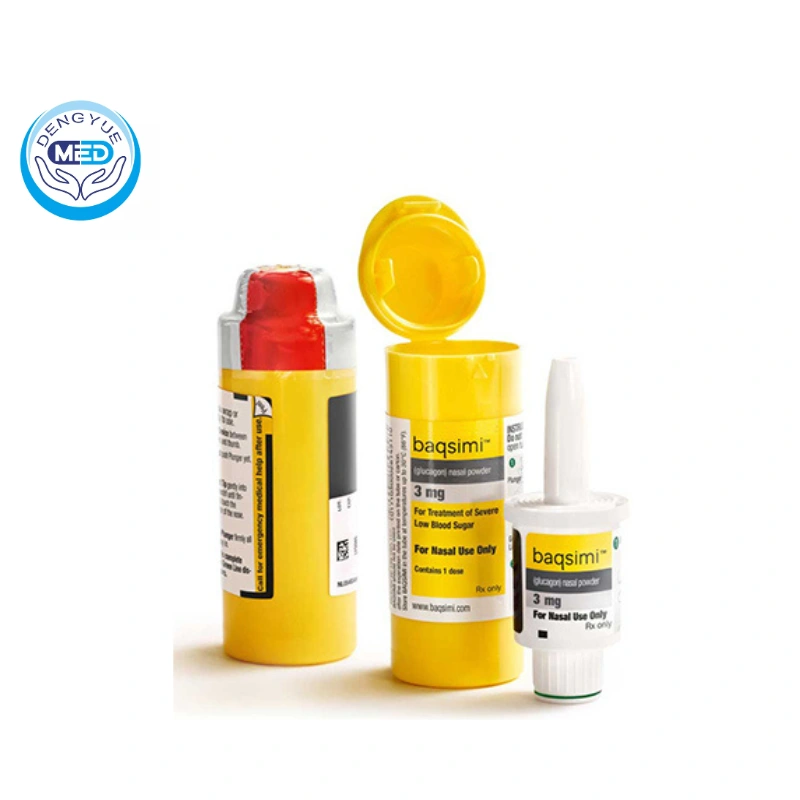
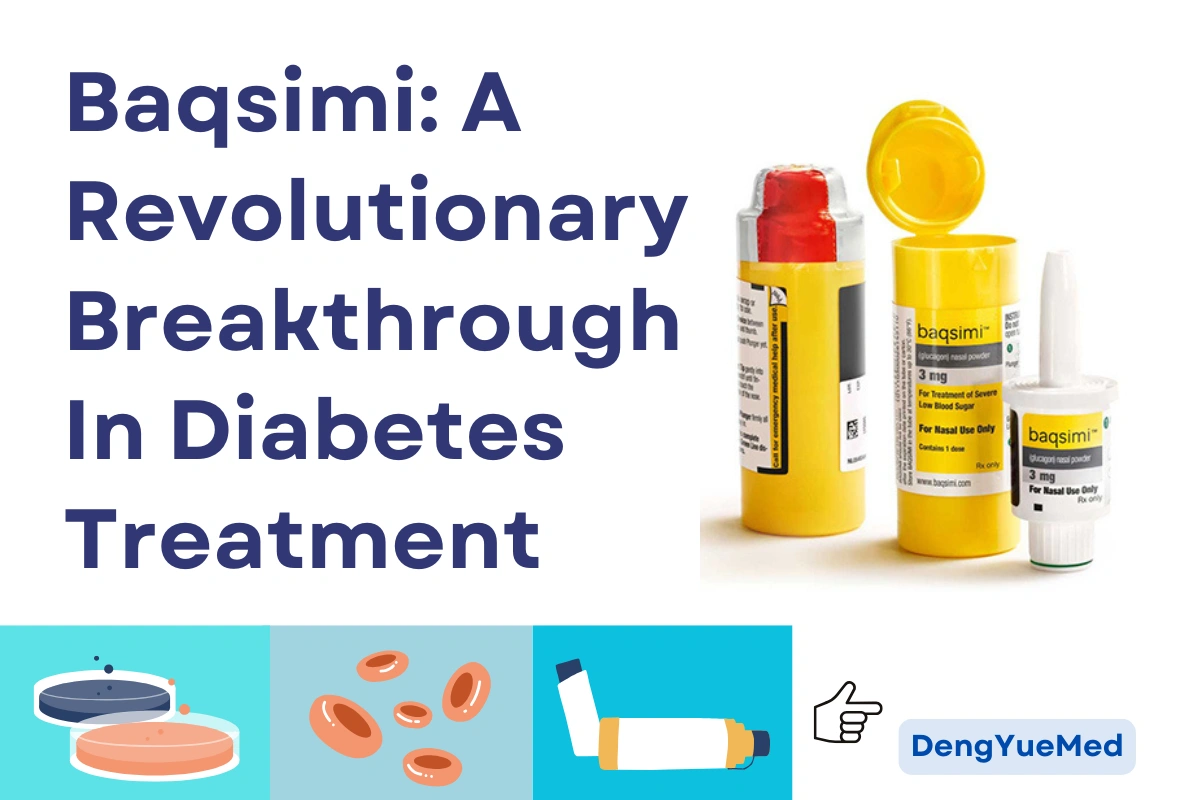
In the field of diabetes treatment, Baqsimi (glucagon nasal powder spray) is emerging as a high-profile innovation.
Developed by Eli Lilly and Company, the drug is administered via nasal spray and offers diabetics a fast and convenient treatment option for severe hypoglycaemia.
Latest News On Baqsimi
FDA Expands Approval: The U.S. Food and Drug Administration (FDA) has expanded the approval of Baqsimi to patients with diabetes aged 1 year and older, after the product was previously only available for patients aged 4 years and older.
China Market Approval: In June 2024, China’s National Drug Administration (NMPA) approved glucagon nasal powder spray for the treatment of severe hypoglycaemic symptoms in diabetic patients aged 4 years and older, making it the first glucagon therapy approved in China that does not require injections.
Market Performance
Sales: Global sales of Baqsimi totalled $139.3 million in 2022.
Sale of Interests: In April 2023, Eli Lilly and Company announced the sale of its global interests in Baqsimi to Amphastar in a transaction totalling $1.075 billion.
Development Progress
Clinical studies: Multiple studies have confirmed that Baqsimi is similar to glucagon injections in terms of efficacy and safety, with 100% treatment success rates.
Glucagon nasal powder spray‘s strengths include its lack of need for injections, rapid absorption, and lower risk of secondary hyperglycaemia.
Mode of administration: Baqsimi is administered via a disposable intranasal dispenser, where the patient simply inserts the tip into the nostril and presses the plunger, without the need to inhale to match, and is administered within 30 seconds, with a success rate of over 90%.
Conclusion
As the first emergency treatment for severe hypoglycaemia that does not require injections, it provides a more convenient emergency treatment option for people with diabetes.
Developed by Eli Lilly, the drug is indicated for the rapid treatment of severe hypoglycaemia (loss of consciousness or inability to eat due to low blood sugar) in patients with type 1 or type 2 diabetes.
The advent of Baqsimi offers a revolutionary treatment option for diabetic patients, especially in the emergency treatment of severe hypoglycaemia.
Its nasal spray delivery simplifies the treatment process and improves patient convenience and adherence.
With the FDA and NMPA approvals, Baqsimi is expected to be more widely used globally to improve the quality of life of diabetic patients.
However, HK DengYue’s efforts to increase the affordability of the medicine are critical to ensuring that more patients benefit from it.
We welcome you to reach out to us to buy Poudre Nasal Glucagon. DengYueMedicine is here to provide detailed medicine information, pricing, and support to ensure a smooth and reliable buying experience.
Feel free to contact us anytime to discuss your needs or ask any questions about the medicine.
Information from DengYueMed, China Drug Import and Export Wholesaler offers this information as a guide, not a substitute for professional medical advice. Consult your doctor before any treatment.



