
Adebrelimab Approval in China: The First Domestic PD-L1 Antibody for Small Cell Lung Cancer
Adebrelimab approval in China marks a significant milestone in the country’s oncology landscape. In 2023, China’s National Medical Products Administration (NMPA) approved adebrelimab injection (Airuili®) for use in combination with standard chemotherapy as a first-line treatment for extensive-stage small cell lung cancer (ES-SCLC).
As reported by DengYue Medicine, this approval goes beyond a single regulatory decision. It represents a defining moment in China’s immuno-oncology development, as adebrelimab becomes the first domestically developed PD-L1 antibody approved in China for small cell lung cancer.
The decision highlights both the progress in addressing long-standing clinical challenges in SCLC and the growing role of homegrown innovation in shaping modern cancer care.
Clinical Background—Unmet Needs and Therapeutic Challenges in Small Cell Lung Cancer
Small cell lung cancer (SCLC) accounts for approximately 15% of all lung cancer cases worldwide, yet it contributes disproportionately to lung cancer–related mortality.
❗ The disease is characterized by rapid tumor proliferation, early metastatic spread, and a high propensity for relapse, making it one of the most aggressive and clinically challenging thoracic malignancies.
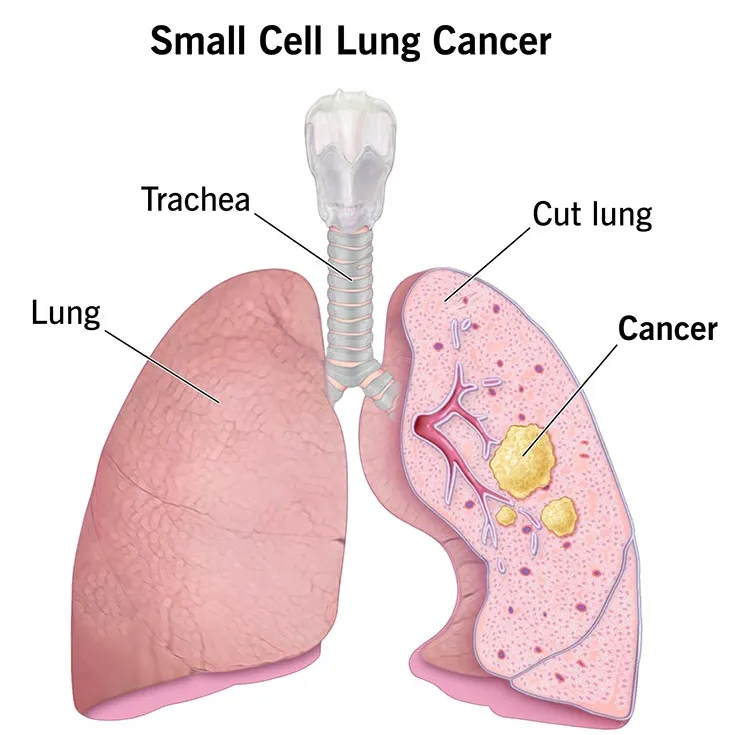
For decades, platinum-based chemotherapy in combination with etoposide remained the cornerstone of treatment for both limited-stage and extensive-stage SCLC. Although initial response rates to chemotherapy are often high, these responses are typically short-lived.
The majority of patients experience disease progression within months, and long-term survival outcomes have shown little improvement over time, underscoring the limitations of cytotoxic therapy alone.
The emergence of immune checkpoint inhibitors has transformed the management of multiple solid tumors, particularly non-small cell lung cancer (NSCLC).
❗ In contrast, therapeutic progress in SCLC has historically lagged behind, reflecting the disease’s biological complexity, rapid clinical course, and the absence of reliable predictive biomarkers. These factors have made drug development in SCLC especially challenging on a global scale.
👉 In recent years, clinical trials have demonstrated that PD-1 and PD-L1 inhibitors, when added to first-line chemotherapy, can deliver meaningful survival benefits for patients with extensive-stage SCLC, establishing immunotherapy-based combinations as a new treatment paradigm. Despite this progress, the real-world impact of immunotherapy has been uneven across regions.
In China and other emerging markets, access to immune checkpoint inhibitors has largely depended on imported products, raising concerns related to treatment affordability, availability, and long-term sustainability within national healthcare systems. As a result, a significant gap has persisted between global therapeutic advances and their practical implementation in routine clinical care.
Against this backdrop, the development of effective, evidence-based domestic immunotherapies has become increasingly important. There is a clear need for treatments that not only demonstrate clinical benefit in rigorous trials but also align with local healthcare realities—creating the foundation for broader and more equitable access to innovative cancer therapies.
Adebrelimab—Mechanism, Indication, and Clinical Significance
Against the backdrop of persistent unmet clinical needs and limited therapeutic breakthroughs in small cell lung cancer, Adebrelimab approval in China represents a meaningful shift in treatment strategy.
Its development reflects a deliberate effort to integrate immunotherapy into frontline SCLC management through robust clinical evidence and domestically driven innovation.
Mechanism of Action: Targeting the PD-1/PD-L1 Immune Checkpoint
Airuili is a humanized IgG4 κappa monoclonal antibody targeting programmed death-ligand 1 (PD-L1), designed to counteract immune escape mechanisms that play a central role in the progression of small cell lung cancer.
PD-L1 is expressed within the tumor microenvironment, where its interaction with the programmed death-1 (PD-1) receptor on activated T cells leads to immune suppression and diminished anti-tumor activity.
By binding selectively to PD-L1 on tumor cells and antigen-presenting cells, adebrelimab blocks the PD-1/PD-L1 immune checkpoint pathway, thereby releasing tumor-induced immune inhibition.
This mechanism enables cytotoxic T lymphocytes to regain functional activity, sustain immune surveillance, and exert durable anti-tumor effects—addressing a key limitation of chemotherapy-based treatment in SCLC.
As a PD-L1 inhibitor, adebrelimab preserves the PD-1/PD-L2 signaling axis, helping maintain immune homeostasis and reduce excessive immune activation—an important factor in achieving a predictable safety profile in first-line combination therapy.
Approved Indication and Clinical Evidence: Phase III CAPSTONE-1 Trial
In 2023, China’s National Medical Products Administration (NMPA) approved Adebrelimab injection in combination with carboplatin and etoposide for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
This decision formally incorporates PD-L1–based immunotherapy into frontline SCLC management in China, aligning domestic clinical practice with evolving global treatment standards.
The regulatory decision was supported by results from the phase III CAPSTONE-1 clinical trial, a randomized, multicenter study designed to evaluate the clinical benefit of adding adebrelimab to standard platinum–etoposide chemotherapy in previously untreated ES-SCLC patients.
Key features of the CAPSTONE-1 trial include:
- Study design: Phase III, randomized, multicenter clinical trial
- Patient population: Previously untreated adult patients with ES-SCLC
- Treatment arms:
- Adebrelimab plus carboplatin and etoposide
- Platinum-etoposide chemotherapy alone
- Primary and key secondary endpoints: Overall survival (OS) and progression-free survival (PFS)
The study demonstrated that the addition of adebrelimab resulted in statistically significant improvements in both OS and PFS compared with chemotherapy alone.
Safety outcomes observed in CAPSTONE-1 were consistent with the known class effects of PD-L1 inhibitors, including:
- No unexpected or new safety signals
- A manageable immune-related adverse event profile
- Feasibility of combining immunotherapy with chemotherapy in the first-line setting
Together, these findings provide high-level, evidence-based support for the clinical value of PD-L1 blockade in ES-SCLC and form the scientific foundation for adebrelimab’s NMPA approval.
Significance of Adebrelimab Approval in China
The approval of adebrelimab directly addresses a critical unmet need in small-cell lung cancer, a disease area that has seen limited therapeutic innovation for decades.
By establishing a PD-L1–based immunotherapy regimen in the first-line ES-SCLC setting, adebrelimab broadens the scope of evidence-based treatment options available to clinicians and patients.
From a clinical perspective, the significance of adebrelimab lies in several key areas:
- First-line immunotherapy option: Provides clinicians with an additional PD-L1–based regimen supported by phase III evidence for ES-SCLC
- Survival benefit beyond chemotherapy: Expands treatment strategies beyond chemotherapy alone, which has historically delivered short-lived responses
- Treatment diversification: Contributes to a more balanced and resilient SCLC treatment landscape by reducing reliance on a single therapeutic approach
- Clinical feasibility: Demonstrates that immunotherapy–chemotherapy combinations can be safely and effectively implemented in routine frontline care
👉 Collectively, these factors reinforce adebrelimab’s role as a clinically meaningful advancement in a disease with long-standing therapeutic limitations.
Beyond its role in patient care, adebrelimab represents an important milestone in the evolution of China’s immuno-oncology ecosystem.
✨ As the first PD-L1 antibody independently developed in China to receive regulatory approval for small cell lung cancer, its success reflects measurable progress across the full drug development continuum.
The broader significance of adebrelimab includes:
- Advancement in antibody engineering: Demonstrates domestic capability in developing high-quality, humanized monoclonal antibodies
- Late-stage clinical development: Validates the ability to conduct large, randomized phase III trials that meet regulatory-grade standards
- Regulatory maturity: Highlights the alignment of domestic development programs with international regulatory expectations
- Healthcare system impact: Supports improved accessibility and long-term sustainability of immunotherapy by reducing dependence on imported products
Taken together, the approval of adebrelimab signals a transition from technology adoption toward innovation ownership, with implications that extend beyond a single product to the broader future of cancer drug development in China.
Strategic Outlook—From Regulatory Approval to Real-World Impact
Adebrelimab approval in China marks more than the introduction of a new treatment option—it reflects a broader shift in how innovation, clinical evidence, and healthcare delivery intersect in the management of small cell lung cancer. As immunotherapy continues to redefine first-line treatment paradigms, the focus is increasingly moving beyond regulatory milestones toward real-world implementation and long-term clinical value.
Looking ahead, the impact of adebrelimab will be shaped not only by its clinical performance but also by how effectively it is incorporated into everyday practice. Continued data generation, post-marketing research, and real-world evidence will be essential to refining patient selection, optimizing treatment sequencing, and maximizing long-term benefit.
As a global pharma partner, DengYue continues to support the translation of innovative therapies into real-world clinical practice through integrated pharmaceutical supply chain solutions and a globally connected distribution network.



