What Sets Saibeixin Pazopanib Tablets Apart: 5 Key Features
When it comes to domestic generic drugs, many people may instinctively associate them with stereotypes such as “cheap substitutes,” “second-choice options,” or “compromised efficacy.”
However, Saibeixin Pazopanib Tablets stand as a clear example that challenges this conventional mindset.
They are by no means a simple copy of the imported originator drug Votrient, nor are they a fallback option chosen out of compromise.
In fact, in the treatment of advanced renal cell carcinoma and soft tissue sarcoma—two critical and particularly challenging therapeutic areas—pazopanib itself, whether as the originator or as a high-quality generic, represents a distinct and irreplaceable treatment strategy.
✨ As a global pharmaceutical supplier—Dengyue, through long-term tracking of the clinical efficacy of pazopanib tablets and related scientific information, will next present an in-depth analysis of “what sets saibeixin pazopanib tablets apart.”
Distinctive Advantage One: Pazopanib in Soft Tissue Sarcoma — Evidence from the PALETTE Trial
🔬 Soft tissue sarcoma (STS) is a group of highly heterogeneous rare malignant tumors comprising more than 50 histological subtypes and accounting for approximately 1% of all malignancies.
Owing to its pathological complexity and diverse biological behavior, STS has long been one of the most challenging cancers to treat.
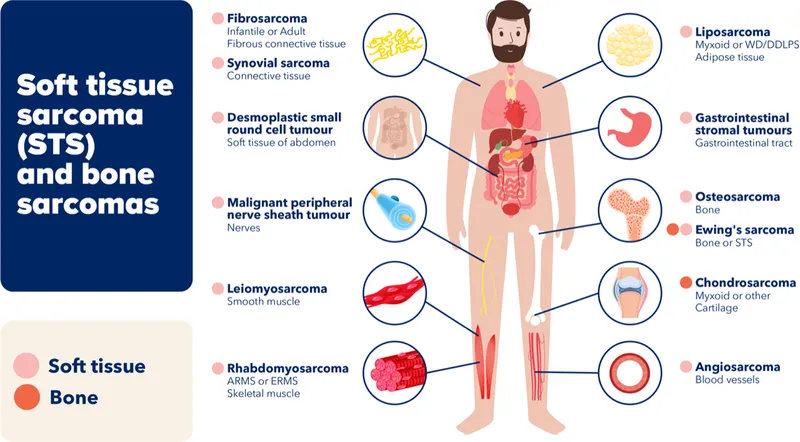
For patients with advanced disease who progress after first-line chemotherapy, therapeutic options have historically been extremely limited, creating a clear and well-recognized “treatment vacuum.”
It was precisely in response to this unmet clinical need that pazopanib (originally marketed as Votrient, with Saibeixin as its high-quality domestic generic) emerged as a landmark solution.
Today, pazopanib is recommended as a standard treatment option by multiple international authoritative guidelines, including NCCN and ESMO, firmly establishing its role in advanced STS.
Robust Evidence Base—The PALETTE Trial Established Its Foundation
👉 The pivotal global, multicenter Phase III PALETTE trial provides decisive evidence for pazopanib’s role in STS.
This study enrolled 369 patients with advanced STS (excluding liposarcoma) who had progressed after prior chemotherapy, particularly anthracycline-based regimens.
Key efficacy outcomes included:
- Median progression-free survival (PFS):
- Pazopanib: 4.6 months
- Placebo: 1.6 months
- Hazard ratio (HR): 0.35; P < 0.001, representing a 65% reduction in the risk of disease progression
- Consistent PFS benefit across major subtypes:
- Leiomyosarcoma: HR = 0.35 (65% risk reduction)
- Synovial sarcoma: HR = 0.42 (58% risk reduction)
- Other subtypes: HR = 0.55 (45% risk reduction)
- Disease control rate (DCR):
- Pazopanib: ~75%
- Placebo: ~39%
This study was the first to clearly demonstrate that a targeted therapy could deliver statistically significant and clinically meaningful benefit in chemotherapy-refractory advanced STS, thereby defining pazopanib as the standard second-line treatment in this setting.
Broad Subtype Coverage with Precise Patient Selection
The approved indication of pazopanib explicitly excludes liposarcoma—a decision that reflects precision oncology, not a limitation.
Clinical benefit has been consistently demonstrated in several common and difficult-to-treat subtypes, including leiomyosarcoma, synovial sarcoma, undifferentiated pleomorphic sarcoma, and angiosarcoma.
✅ For a disease as biologically diverse as STS, this broad yet well-defined activity provides a practical and unified treatment strategy, avoiding the unrealistic challenge of developing a separate drug for every rare subtype.
A Mechanism of Action Aligned with Sarcoma Biology
The growth and metastasis of soft tissue sarcomas are highly dependent on angiogenesis, driven primarily by the VEGF signaling pathway, as well as other tyrosine kinase pathways such as PDGFR.
Pazopanib’s mechanism of action—as a multi-targeted tyrosine kinase inhibitor—allows it to simultaneously inhibit VEGFR-1/2/3, PDGFR-α/β, and c-KIT, effectively disrupting tumor blood supply and growth signaling through multiple pathways.
This “multi-pronged” inhibition is widely considered a key molecular basis for its success in STS.
💊 Importantly, pazopanib is not a chemotherapy agent but a targeted oral therapy, with a well-characterized safety profile and manageable pazopanib side effects through routine monitoring.
✨ In the field of soft tissue sarcoma—where treatment options are scarce—pazopanib has become synonymous with standard second-line therapy after chemotherapy failure.
Its position is supported by rigorous Phase III clinical evidence, proven efficacy across multiple clinically important subtypes, and a mechanism of action that directly addresses the biological drivers of the disease.
Distinctive Advantage Two: Pazopanib in Advanced RCC — COMPARZ Trial and Quality of Life
In the first-line treatment landscape for advanced renal cell carcinoma (RCC), pazopanib occupies a uniquely differentiated position.
Data from the pivotal head-to-head COMPARZ trial demonstrate that pazopanib delivers comparable core efficacy to the comparator therapy in terms of overall survival (28.4 vs 29.3 months) and progression-free survival (8.4 vs 9.5 months), while achieving a higher objective response rate (31% vs 25%).
However, the true clinical distinction lies not in survival curves alone, but in treatment tolerability and patient quality of life—a dimension that increasingly defines value in modern oncology.
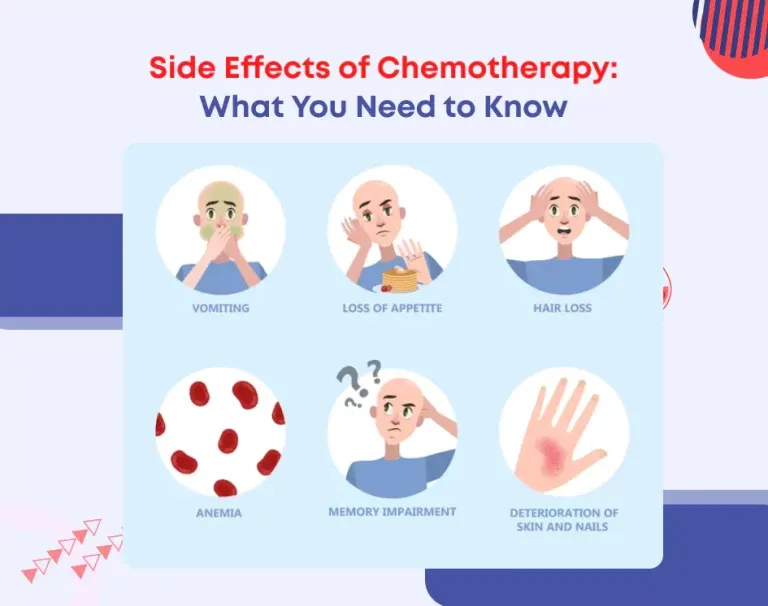
Meaningful Differences in the Treatment Experience
👉 Fatigue management represents one of the most clinically relevant advantages.
In the COMPARZ study, the incidence of grade 3–4 fatigue was 34% with pazopanib, compared with 43% in the comparator group.
In practical terms, this translates to nearly one fewer patient experiencing severe fatigue for every ten treated, a difference that directly impacts daily functioning and independence.
An even more pronounced contrast was observed in hand–foot skin reaction (HFSR).
- Any-grade HFSR occurred in 29% of patients receiving pazopanib, with only 6% experiencing severe reactions
- Versus 50% and 17%, respectively, in the comparator arm
This represents a nearly two-thirds reduction in the risk of severe HFSR, a toxicity known to significantly impair basic activities such as walking, dressing, and manual tasks.
Pazopanib also demonstrated advantages in adverse events affecting nutritional intake, including lower rates of taste disturbances (26% vs 36%) and oral mucositis.
These differences are clinically meaningful, as they influence appetite, nutritional status, and overall treatment tolerance.
Deeper Clinical Implications
✨ These findings reflect more than simply “fewer side effects.” They represent a shift in therapeutic philosophy—one that seeks to maintain equivalent survival benefit while actively protecting quality of life.
Importantly, pazopanib is not a chemotherapy agent but an oral targeted therapy with a well-defined mechanism of action as a multi-target tyrosine kinase inhibitor, which contributes to its differentiated safety profile and predictable pazopanib side effects.
For patients with advanced RCC requiring long-term treatment, this balance means the ability to live better while living longer—maintaining physical strength, social engagement, and independence alongside disease control.
From a clinical practice perspective, improved tolerability supports better adherence, fewer dose interruptions or reductions, and more continuous treatment exposure, all of which may translate into more sustained clinical benefit.
Moreover, preserved quality of life enables patients to maintain work capacity, social roles, and psychological stability.
Distinctive Advantage Three: Pazopanib Mechanism of Action — Multi-Target TKI Synergy
What fundamentally differentiates pazopanib from many single-target or narrow-spectrum targeted therapies is its multi-target tyrosine kinase inhibition profile.
This characteristic elevates its anticancer strategy from traditional single-pathway blockade to a more comprehensive and systemic multi-pathway synergistic inhibition.
Multiple Mechanisms Form a Three-Dimensional Antitumor Network
Pazopanib simultaneously and precisely acts on several key signaling pathways that drive tumor initiation and progression:
- Potent anti-angiogenic activity (VEGFR-1/2/3 inhibition)
By targeting vascular endothelial growth factor receptors, pazopanib effectively suppresses tumor neovascularization, disrupting the tumor’s blood supply and achieving the fundamental goal of “starving” the tumor.
- Direct inhibition of tumor cell proliferation (PDGFR-α/β inhibition)
Platelet-derived growth factor receptors play a critical role in tumor cell growth and division.
Inhibiting PDGFR allows pazopanib to act directly on tumor cells themselves, restraining malignant proliferation.
- Disruption of tumor microenvironment and cell–cell communication (FGFR-1/3 inhibition)
Fibroblast growth factor receptors are involved not only in tumor growth but also in signaling interactions between tumor cells and their surrounding microenvironment.
Blocking this pathway interferes with the tumor’s supportive signaling network.
- Targeting key oncogenic drivers in specific subtypes (c-KIT inhibition)
c-KIT is a well-established driver mutation in certain sarcoma subtypes, such as gastrointestinal stromal tumors.
Pazopanib’s activity against c-KIT enables more direct and precise inhibition in tumors dependent on this pathway.
Deeper Clinical Implications of Multi-Target Synergy
This multi-target, multi-level strategy translates into clinically meaningful advantages beyond what single-target agents can offer:
- Reduced resistance and prolonged disease control
Tumor cells can rapidly evade single-target inhibition by activating alternative signaling pathways.
Pazopanib’s broad-spectrum inhibition acts like “multiple locks” placed simultaneously on escape routes, making adaptive resistance more difficult and potentially extending the duration of clinical benefit.
- Addressing tumor heterogeneity and improving treatment success
In highly heterogeneous tumors such as soft tissue sarcoma, different tumor cell subpopulations may rely on distinct survival pathways.
A multi-target agent functions like a “combination strike,” covering multiple growth dependencies at once and producing broader antitumor activity—helping explain why pazopanib demonstrates efficacy across multiple sarcoma subtypes.
- Providing therapeutic opportunities in heavily pretreated, complex cases
Patients with advanced sarcoma who had failed multiple prior lines of therapy—including chemotherapy and other targeted agents—switching to pazopanib still resulted in disease stabilization in a subset of patients.
Investigators attributed this benefit to pazopanib’s unique multi-target mechanism of action, which may inhibit previously untargeted or resistance-associated bypass pathways.
Saibeixin’s mechanism of action is not a simple accumulation of targets, but a synergistic, system-level anticancer strategy
It simultaneously attacks tumors at their source (angiogenesis), at the cellular level (tumor proliferation), within the tumor microenvironment, and through precise inhibition of subtype-specific driver targets.
Distinctive Advantage Four: Saibeixin Pazopanib as a High-Quality Generic — Cost and Accessibility
As a high-quality generic that has passed China’s Generic Drug Consistency Evaluation, Saibeixin® Pazopanib Tablets demonstrate their core value by successfully translating a globally recognized standard therapy into a cost-effective and widely accessible treatment option, achieving a meaningful breakthrough in treatment accessibility.
Economic Accessibility: Significantly Lowering the Global Treatment Barrier
The introduction of Saibeixin fundamentally reshapes the economic accessibility of pazopanib therapy.
💰 The monthly cost of the imported originator drug is approximately $2,000–2,800, and even with insurance coverage in some regions, patients may still face hundreds to thousands of dollars in out-of-pocket expenses.
💰 By contrast, Saibeixin, as a high-quality generic, reduces the monthly treatment cost to approximately $400–700.
After reimbursement, patients’ actual out-of-pocket expenses can be further lowered to around $140–280 per month.
🙂 This substantial price difference translates into an annual reduction in treatment burden of approximately $15,000–20,000.
For patients requiring long-term therapy, this is not merely a financial relief, but a critical factor in maintaining treatment adherence and continuity.
Under the premise of equivalent efficacy, Saibeixin enables more patients to receive standardized and uninterrupted pazopanib treatment.
Quality Assurance: A Solid Foundation of Proven Equivalence
Saibeixin has successfully passed the National Medical Products Administration (NMPA) consistency evaluation, a rigorous regulatory framework designed to ensure both quality and clinical reliability. This evaluation requires that:
- Bioequivalence be demonstrated through pharmacokinetic studies, confirming that absorption rate and extent are highly consistent with the originator drug
- Manufacturing processes and quality standards meet internationally advanced benchmarks
- Clinical data support equivalence in efficacy and safety compared with the originator product
This certification provides strong scientific and regulatory assurance that patients gain improved affordability without compromising efficacy or safety.
Broader Social Value: Advancing Equity in Global Healthcare Access
Beyond individual patient benefit, the accessibility breakthrough achieved by Saibeixin carries significant societal implications:
- Improved treatment equity: enabling patients across different economic backgrounds to access internationally accepted standard therapies, narrowing disparities in healthcare resource distribution
- Optimized healthcare system efficiency: reducing overall treatment costs while maintaining efficacy, thereby enhancing the sustainability of healthcare and insurance systems
- Better treatment outcomes: reduced financial pressure allows patients to adhere more confidently to recommended therapy, supporting long-term disease control and quality of life
- Industry advancement: the development of high-quality generics promotes technological progress and healthy competition within the pharmaceutical sector, ultimately expanding affordable treatment options for patients worldwide
✨ These dimensions of affordability, quality assurance, and social impact collectively explain what sets Saibeixin Pazopanib Tablets apart.
As a non-chemotherapy, oral targeted therapy with a well-established clinical profile, pazopanib’s value is amplified when paired with genuine accessibility.
Distinctive Advantage Five: Pazopanib Side Effects and Safety Management
One notable advantage that distinguishes pazopanib (including Saibeixin® Pazopanib Tablets) from many newer targeted therapies is that, through long-term clinical use, it has established a clear, standardized system for adverse event monitoring and management.
This well-defined “management roadmap” makes the treatment course highly predictable and controllable, significantly enhancing long-term safety as well as efficiency in physician–patient collaboration.
A Systematic and Standardized Management Framework
The pazopanib side effects profile is well characterized and primarily involves hepatic function changes, blood pressure elevation, and gastrointestinal reactions—all of which are measurable and routinely monitorable.
Based on extensive clinical experience and evidence-based guidelines, standardized management pathways have been developed.
Key elements of this framework include:
- Proactive liver function monitoring
- Active blood pressure management strategies
- Standardized management of other common adverse events
The Clinical Value of Structured Safety Management
This clearly defined management roadmap delivers multiple clinical benefits:
- Risk mitigation: Routine monitoring and early intervention minimize the likelihood of severe complications, such as serious liver injury or uncontrolled hypertension.
- Enhanced physician–patient collaboration: Clear expectations and predefined solutions reduce patient anxiety and uncertainty.
- Treatment continuity and durability: Standardized management ensures that adverse events do not unnecessarily interrupt effective therapy.
Pazopanib (Saibeixin) provides not only an effective therapeutic agent, but also a validated “user manual” for safe and sustained use.
✨ Its clear and standardized management roadmap transforms common challenges of targeted therapy into structured, executable clinical procedures.
By pairing improved accessibility with consistent safety oversight, what sets Saibeixin Pazopanib Tablets apart is their ability to deliver high-quality, sustainable cancer treatment without compromising patient safety—a cornerstone of modern oncology care.
Conclusion: A “Smart Choice” in Modern Cancer Therapy
The value of Saibeixin® Pazopanib Tablets cannot be adequately captured by the simple label of a “generic drug“.
👉 Instead, they represent an important direction in modern cancer treatment—a shift away from the mindset of “eliminating the tumor at any cost” toward a more balanced approach that seeks to control disease effectively while preserving patients quality of life.
Saibeixin® Pazopanib Tablets are a high-quality generic of pazopanib, a guideline-recommended targeted therapy for the treatment of advanced renal cell carcinoma and soft tissue sarcoma.
They offer equivalent clinical efficacy, a well-established safety management framework, and greater affordability, making long-term treatment more accessible.
As emphasized by DengYue Medicine, for appropriately selected patients, Saibeixin addresses a central challenge of modern oncology: delivering standard-of-care therapy without compromising sustainability or quality of life.
It is a thoughtful and informed decision—one that reflects what sets Saibeixin Pazopanib Tablets apart in today’s oncology landscape.
FAQ about What Sets Saibeixin Pazopanib Tablets Apart
What is the drug pazopanib used for?
Pazopanib is an antineoplastic agent used in the treatment of advanced renal cell cancer and advanced soft tissue sarcoma in patients with prior chemotherapy.
How long can you stay on pazopanib?
You will usually carry on taking pazopanib for as long as it is working for you.
What is the success rate of pazopanib?
Response to pazopanib therapy: None of the patients showed a complete response.
Does pazopanib cause weight loss?
Yes, it will experience weight loss and lack of fluid in your body (dehydration).
So you should drink plenty of fluid while taking pazopanib.