
Redefining Late-Line Therapy in Advanced GIST: What is The Mechanism of Action of Ripretinib?
The treatment of advanced gastrointestinal stromal tumors (GIST) is a long and arduous race against genetic mutations.
💊 Patients typically follow a standard sequential treatment pathway: first-line imatinib, second-line sunitinib, and third-line regorafenib.
☹️ However, tumor cells are highly adaptive, continuously evolving and developing new resistance mutations. When third-line therapy also fails, many patients and their families are left facing a sense of having “no options left.”
Conventional chemotherapy offers minimal benefit in GIST, and for a long time, effective late-line therapies were scarce, leading to a sharp decline in survival.
It was against this challenging clinical backdrop that ripretinib emerged like a beam of light cutting through the darkness.
But what is the mechanism of action of ripretinib that allows it to overcome such complex resistance?
As highlighted through real-world treatment observations and supply experience from Dengyue, a pharmaceutical wholesaler engaged across multiple therapeutic areas, Ripretinib is not merely a new medication; it represents an evolution in treatment philosophy.
By targeting the most fundamental molecular switch governing kinase activation, it offers a rational and durable response to tumor evolution..
👉 This article explores the role of ripretinib in redefining late-line therapy for advanced GIST and examines how this so-called “molecular lock” exerts its critical clinical impact.
Driver Genes and the Evolution of GIST Treatment
To understand how ripretinib emerged as a true “game changer,” we must first clearly define the “impasse” it was designed to break—the complex and formidable challenge that patients with advanced GIST ultimately face after a long course of treatment.
The Nature of GIST: A War Triggered by Genetic “Betrayal”
Gastrointestinal stromal tumors (GIST) are relatively “distinct” among malignancies.
They are not conventional gastric or colorectal cancers, which arise from epithelial cells. Instead, GIST originate from mesenchymal cells within the wall of the gastrointestinal tract—cells that can be thought of as the structural “scaffolding” that supports and connects tissues.
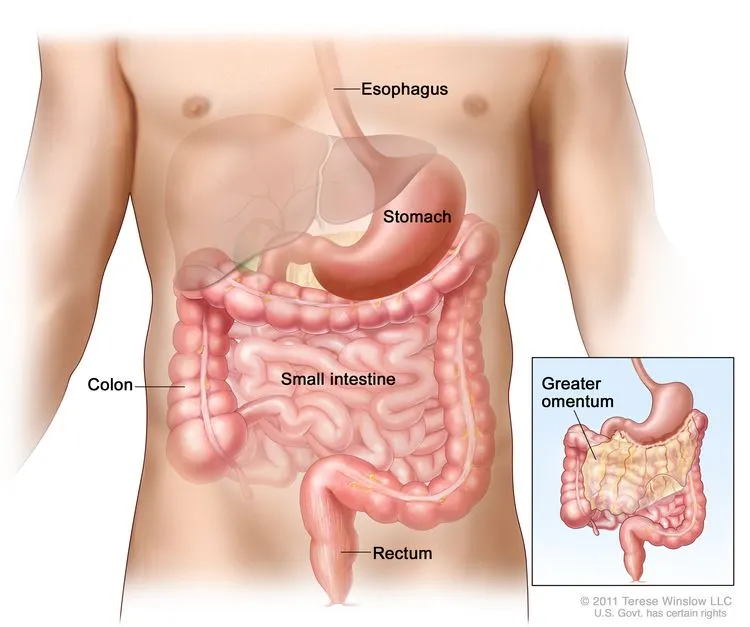
This unique origin fundamentally shapes both the biological behavior of GIST and the therapeutic strategies used to treat it.
🧬 The “betrayal” of driver genes (core mechanism): Approximately 85–90% of GIST cases are driven by specific gain-of-function mutations in the KIT or PDGFRA genes, which serve as the central molecular engines of tumor initiation and progression.
🔬 These two genes function much like the “master switch” and “accelerator pedal” of cellular growth signaling.
Under normal conditions, their activity is precisely regulated according to the body’s needs. Once mutated, however, it is as if the accelerator pedal becomes jammed to the floor—growth signals are continuously and uncontrollably transmitted, driving unchecked cellular proliferation and ultimately tumor formation.
Paradoxically, this clearly defined, dominant driver represents a silver lining.
✨ It has made GIST one of the most successful models for targeted therapy, allowing researchers to design drugs that precisely shut down this specific “malfunctioning accelerator.”
The “Relay Race” of Targeted Therapy and the Challenge of Drug Resistance
Since the introduction of the first targeted agent, imatinib (Gleevec), in 2002, the treatment of GIST has entered a revolutionary era.
The therapeutic journey for patients resembles a race against time—a relay race of targeted therapies.
- The First Leg (First Line): Imatinib—The “Miracle Drug” That Opened a New Era
- Mechanism of action: Imatinib precisely binds to the “power switch” of the KIT/PDGFRA proteins—the ATP-binding pocket—thereby blocking their kinase activity.
- Landmark achievements: Imatinib dramatically extended the median overall survival of patients with advanced GIST from less than 2 years to more than 5 years. Median progression-free survival (PFS) reached 2–3 years, allowing many patients to live long-term with controlled disease and markedly improved quality of life.
- The Second Leg (Second Line): Sunitinib—The First “Breakthrough Weapon” After Resistance
- Clinical background: However, after an average of approximately two years, more than half of patients develop resistance to imatinib.
- Mechanism and outcomes: As a multitargeted tyrosine kinase inhibitor, sunitinib suppresses multiple kinases, including KIT.
✅ When imatinib fails, sunitinib can regain tumor control, providing a median PFS of approximately 6–9 months.
- The Third Leg (Third Line): Regorafenib—A Further Line of Defense
- Clinical role: Regorafenib enters the treatment sequence after resistance to sunitinib, once again buying valuable time for patients.
- Key evidence: The phase III GRID study demonstrated that regorafenib reduced the risk of disease progression by 73% compared with placebo, with a median PFS of approximately 4.8 months.
Tumors are formidable and adaptive adversaries.
Under the selective pressure of targeted therapies, they evolve secondary resistance mutations, particularly within the ATP-binding pocket (exons 13/14) and the activation loop (exons 17/18) of the KIT gene.
This process is akin to a lock (the kinase) repeatedly changing its internal mechanism, rendering the original keys (earlier targeted agents) completely ineffective.
☹️ By the time patients reached fourth-line therapy, the clinical landscape had long lacked effective agents capable of addressing these complex resistance mutations.
Breaking the Deadlock: How Ripretinib “Locks Down” Tumor Resistance Pathways
The development of ripretinib, commercially known as Qinlock®, was driven by a deeper understanding of the fundamental mechanisms underlying drug resistance in gastrointestinal stromal tumors (GIST).
✨ Rather than competing with tumor cells at an ever-changing active site, ripretinib adopts a more foundational and strategic approach—one that directly targets the molecular process governing kinase activation itself.
This innovation defines the role of ripretinib as a true paradigm-shifter in late-line GIST therapy.
Limitations of Conventional TKIs: A Struggle at the “Keyhole”
Traditional tyrosine kinase inhibitors (TKIs), such as imatinib, exert their effects by binding to the ATP-binding pocket of KIT or PDGFRA kinases when these proteins adopt an active conformation.

Imatinib Mesylate Tablets | Leukaemia
- Generic Name/Brand Name: Imatinib Mesylate Tablets/Gleevec
- Indications: Leukaemia
- Dosage Form: White powder
- Specification: Available in tablet strengths of 100 mg and 400 mg.
This strategy proved revolutionary at the time, delivering unprecedented clinical benefits in early-line treatment.
However, this mechanism is inherently vulnerable.
Under prolonged therapeutic pressure, secondary mutations frequently arise within the ATP-binding pocket or the activation loop, altering the structural “keyhole” into which these drugs must fit.
🤨 Once this precise molecular fit is disrupted, drug binding weakens and resistance inevitably develops.
In advanced GIST, this limitation becomes increasingly pronounced as multiple resistant clones emerge simultaneously.
Ripretinib’s Mechanism of Action
- Targeting the Molecular Switch
The ripretinib mechanism of action represents a fundamental departure from this traditional paradigm.
✨ Ripretinib binds with high affinity to a distinct regulatory region of KIT and PDGFRA known as the switch pocket—a structural element that controls the transition of the kinase from an inactive to an active state.
.webp)
Rather than attempting to shut down a kinase that is already “on,” ripretinib effectively prevents activation from occurring in the first place.
✨ By stabilizing the kinase in its inactive conformation and blocking the conformational shift required for activation, ripretinib functions as a molecular lock on the kinase activation process.
This “switch-control” strategy explains the broad inhibitory activity of Qinlock® (ripretinib) against a wide spectrum of KIT and PDGFRA mutations, including those located in the ATP-binding pocket (exons 13/14) and the activation loop (exons 17/18).
- Dual-Level Inhibition: Structural Stability and Dynamic Control
At the molecular level, ripretinib achieves dual inhibition:
- Structurally, it stabilizes KIT and PDGFRA in an inactive state, suppressing baseline kinase signaling.
- Dynamically, it blocks the conformational transition required for reactivation, eliminating the kinase’s ability to respond to activating signals.
✅ This dual-layer control makes it far more difficult for tumor cells to escape through single-point mutations, providing a robust mechanistic foundation for ripretinib’s activity in heavily pretreated, mutation-diverse GIST.
- Designed for Real-World Tumor Complexity
Advanced GIST is rarely driven by a single resistant mutation. Instead, it is characterized by polyclonal resistance, with multiple tumor subpopulations harboring different secondary mutations.
Unlike earlier TKIs that target specific mutation subsets, ripretinib was designed to suppress the common activation pathway shared by diverse resistant clones.
👉 This ability to address molecular heterogeneity is central to the intrigue of ripretinib—its appeal lies not only in potency, but in its conceptual elegance: it targets the process of resistance itself, rather than chasing individual mutations as they arise.
- Clinical Relevance and Safety Considerations
In clinical use, ripretinib has demonstrated a manageable safety profile consistent with its targeted mechanism.
Ripretinib side effects most commonly include alopecia, fatigue, gastrointestinal symptoms, and hand–foot skin reactions, with most adverse events being predictable and clinically manageable through monitoring and supportive care.
Taken together, the balance between broad antitumor activity and tolerability further reinforces ripretinib’s role as a cornerstone therapy in late-line GIST management.
By redefining how kinase activity is controlled at a structural level, Ripretinib (Qinlock®) moves GIST treatment beyond incremental drug development toward true mechanism-driven innovation.
🟢 Its ability to lock down resistance pathways explains not only its clinical efficacy, but also its growing recognition as a critical advance in the management of advanced GIST.
Defining Evidence That Reshaped the Landscape — Key Clinical Trial Data
Even the most elegant therapeutic design must ultimately prove its value on the real clinical battlefield.
🔬 The unique “switch-pocket” inhibitory strategy of ripretinib is not merely a theoretical concept; it has been translated into tangible survival benefits through several landmark clinical studies, fundamentally reshaping the treatment landscape of advanced GIST.
These data provide the most compelling validation of the critical role of ripretinib.
1. Establishing the Fourth-Line Standard of Care: The INVICTUS Trial — Creating a Path Where None Existed
The INVICTUS trial addressed one of the most challenging patient populations in clinical oncology: individuals with advanced GIST whose disease had progressed after prior treatment with imatinib, sunitinib, and regorafenib.
- Striking efficacy difference: Results demonstrated a median progression-free survival (PFS) of 6.3 months in the ripretinib group, compared with just 1.0 month in the placebo group.
- Fundamental improvement in overall survival: Even more compelling, the final analysis revealed a median overall survival (OS) of 18.2 months for patients receiving ripretinib—nearly three times longer than that observed in the placebo group (6.3 months).
✅ In the late-line treatment of advanced malignancies, such a definitive OS benefit represents the gold standard for evaluating clinical value.
The INVICTUS trial conclusively demonstrated that when conventional treatment options have been exhausted, ripretinib can deliver survival prolongation that is both statistically robust and clinically meaningful.
On the basis of these data, ripretinib has been uniformly recommended by international treatment guidelines, firmly establishing its position as the cornerstone standard of care in fourth-line therapy for advanced GIST.
2. Highlighting Therapeutic Value Beyond Efficacy: The INTRIGUE Trial — Balancing Tumor Control and Quality of Life
Although ripretinib did not meet the primary endpoint of superior PFS compared with sunitinib in the overall second-line population, the INTRIGUE trial revealed another critical dimension of its value: markedly improved tolerability and preservation of quality of life.
- Significant safety advantage: The incidence of treatment-related adverse events of grade ≥3 was 22.4% in the ripretinib group, substantially lower than the 48.1% observed with sunitinib. This represents a reduction of more than 50% in the risk of severe toxicity.
- Key adverse event comparisons: Differences were particularly pronounced for side effects with major impact on daily functioning:
- Hypertension (grade ≥3): Ripretinib 0.9% vs sunitinib 10.8%
- Hand–foot skin reaction (grade ≥3): Ripretinib 0.9% vs sunitinib 9.7%
These findings confirm that ripretinib offers an important option that is both effective and gentler.
👨👩👧👦 For elderly patients, those with compromised performance status, or individuals unable to tolerate the intensive toxicities of traditional TKIs, ripretinib enables sustained disease control while preserving quality of life—achieving a critical balance between efficacy and tolerability.
3. Precision Targeting of a Resistant Stronghold: PDGFRA D842V Mutation — From “Untreatable” to Precisely Targeted
Approximately 5–10% of patients with GIST harbor the PDGFRA D842V mutation, a subtype characterized by primary resistance to imatinib and historically associated with a lack of effective therapeutic options.
Early-phase studies revealed remarkable activity of ripretinib against this mutation.
In the PDGFRA D842V cohort of the pivotal phase I study (the precursor to INVICTUS), the objective response rate (ORR) reached 84%, with responses that were both deep and durable.
✨ Based on these compelling data, ripretinib received Breakthrough Therapy Designation from the U.S. FDA and was subsequently approved, becoming the first—and currently the only—targeted therapy specifically indicated for PDGFRA D842V–mutant GIST.
In summary, ripretinib has demonstrated its irreplaceable value across multiple dimensions—from serving as a solid fourth-line foundation for patients with multi-drug resistance, to offering a more tolerable treatment alternative for broader patient populations, and to precisely overcoming a previously insurmountable resistance mutation.
Conclusion: A Transformed Landscape, a Promising Future
The emergence of ripretinib marks a pivotal shift in the treatment of gastrointestinal stromal tumors (GIST)—from a strategy centered on simple sequential drug use to an era of precision planning and resistance-focused therapeutic management.
For patients and their families, this progress means that even after multiple lines of therapy have failed, effective and tolerable treatment options remain available, and hope is far from extinguished.
👀 Looking ahead, deeper insights into resistance mechanisms, along with ongoing investigations into combination strategies involving ripretinib, are expected to further broaden and smooth the treatment pathway for advanced GIST.
HongKong DengYueMedicine thinks that the evolution of ripretinib exemplifies how mechanism-driven innovation can be translated into real-world clinical impact.
As science continues to advance, the outlook for patients with advanced GIST grows ever more promising.
FAQ about What is The Mechanism of Action of Ripretinib
What is ripretinib used for?
Ripretinib is used to treat gastrointestinal stromal tumor (GIST) in patients who have received treatment with at least 3 other medicines.
Is ripretinib a TKI?
Yes, ripretinib is a switch control TKI.
Is ripretinib chemotherapy?
No, it’s a targeted therapy. In summary, QINLOCK, or ripretinib, is a TKI for advanced GIST, but it works a little bit differently than the prior TKIs.
How much does ripretinib cost?
1 mg about $60 in stock



