What Is Taltz Ixekizumab Used For? A Precision Biologic That Targets IL-17A–Driven Inflammation
What is Taltz ixekizumab used for? This is a common question among patients and healthcare professionals seeking clearer information about modern biologic therapies for immune-mediated inflammatory diseases.
As treatment strategies continue to evolve toward targeted and precision-based approaches, understanding how biologic medicines like ixekizumab work—and who they are intended for—has become increasingly important.
As a worldwide pharma supplier, we aim to provide accurate, up-to-date drug information to support informed decision-making across the healthcare supply chain.
In this article, we offer a clear and patient-friendly overview of ixekizumab injection (Taltz®), including its mechanism of action, approved indications, how it differs from traditional therapies, and key considerations for use.
Ixekizumab: A Targeted Biologic and Its Mechanism of Action
Before exploring how ixekizumab is used in clinical practice, it is important to first understand what ixekizumab injection is and how it works at a biological level. As a targeted biologic therapy, its mechanism of action is closely linked to the underlying immune pathways involved in chronic inflammatory diseases.
The following section provides a clear overview of the drug’s nature and the scientific principles behind its effects.
What Is Ixekizumab Injection?
Ixekizumab injection (brand name: Taltz®) is a biologic therapy used to treat certain immune-mediated inflammatory diseases. It is a a humanized IgG4 monoclonal antibody targeting IL-17A produced through advanced biotechnology and administered by subcutaneous injection.
Unlike traditional chemically synthesized oral medications, ixekizumab is a protein-based medicine designed to recognize and bind to a specific target involved in inflammation.
✨ By focusing on a single, well-defined immune pathway, ixekizumab represents a more targeted approach to disease management and is often considered when conventional treatments do not provide adequate disease control.
How Does Ixekizumab Work?
🔴 Ixekizumab works by selectively targeting interleukin-17A (IL-17A), a key cytokine that plays a central role in driving chronic inflammation in several immune-mediated diseases.
IL-17A acts as an important signaling molecule in the immune system. When produced in excess, it can promote persistent inflammation, leading to clinical manifestations such as skin lesions, joint pain, stiffness, and progressive tissue damage.
This dysregulated inflammatory response is a key feature of conditions such as plaque psoriasis and ankylosing spondylitis.
Ixekizumab binds specifically to IL-17A and blocks its interaction with inflammatory signaling pathways, thereby reducing the downstream immune response. By interrupting this process at a critical point, ixekizumab helps modulate abnormal inflammation while maintaining overall immune function.
This targeted mechanism of action underpins the role of ixekizumab in modern precision medicine and provides a scientific basis for its use in the long-term management of immune-mediated inflammatory diseases.
Where Is Ixekizumab Used—and Why Do These Indications Matter?
Immune-mediated inflammatory diseases such as plaque psoriasis and ankylosing spondylitis are chronic, progressive conditions driven by persistent immune dysregulation.
Despite advances in systemic therapies, many patients continue to experience inadequate disease control, cumulative organ damage, and reduced quality of life, highlighting a clear need for more targeted and durable treatment options.
By selectively inhibiting IL-17A, a key cytokine shared across multiple inflammatory pathways, ixekizumab has gradually expanded its clinical role from dermatology into rheumatology, reflecting both evolving scientific understanding and unmet clinical needs.
According to the National Medical Products Administration (NMPA)–approved labeling in China, ixekizumab injection is currently indicated for the following conditions:
1️⃣ Moderate to Severe Plaque Psoriasis: Addressing Rapid Disease Control and Long-Term Burden
Plaque psoriasis is more than a skin condition—it is a systemic inflammatory disease associated with pain, itching, psychological distress, and an increased risk of metabolic and cardiovascular comorbidities. For patients with moderate to severe disease, topical therapies are often insufficient, and traditional systemic agents may be limited by delayed onset, cumulative toxicity, or tolerability issues.
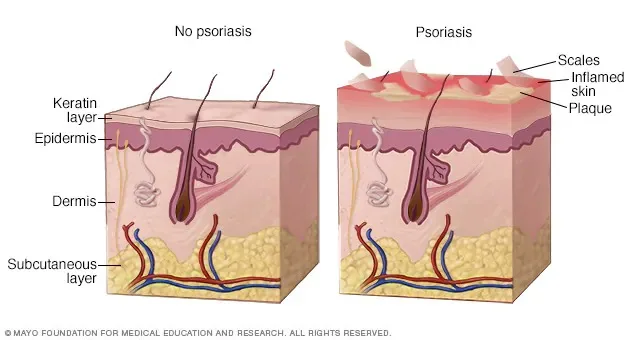
Ixekizumab is approved for adult patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Clinical trials have shown that ixekizumab provides rapid onset of action and high levels of skin clearance, with clinical improvement observed as early as 1–2 weeks.
A substantial proportion of patients achieved PASI 75 and PASI 90 responses by week 12, with some demonstrating meaningful improvement within the first few weeks of treatment. Importantly, these responses were maintained with continued therapy, supporting its use in long-term disease management.
By directly targeting IL-17A, ixekizumab intervenes at a central driver of keratinocyte hyperproliferation and cutaneous inflammation, offering patients both fast symptom relief and sustained disease control.
2️⃣ Active Ankylosing Spondylitis: Why This Indication Came Later
Ankylosing spondylitis (AS) is a chronic inflammatory disease primarily affecting the axial skeleton, leading to persistent back pain, stiffness, reduced mobility, and irreversible structural damage if not adequately controlled.
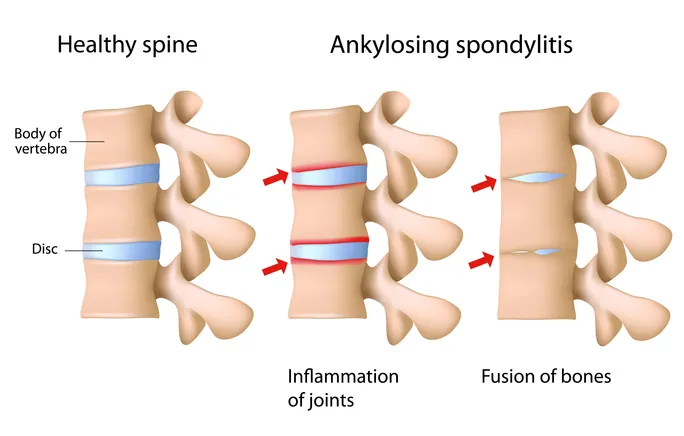
🤔 Historically, treatment options were limited to NSAIDs and later TNF inhibitors, leaving a significant subset of patients with insufficient response or intolerance.
Ixekizumab was later approved for active ankylosing spondylitis in adults with an inadequate response to conventional therapy, reflecting both scientific caution and accumulating clinical evidence.
The delayed approval of this indication is closely tied to the stepwise validation of the IL-17A pathway in axial disease. While IL-17A was first established as a dominant driver in psoriasis, its role in spondyloarthritis required dedicated long-term studies to confirm efficacy, functional improvement, and safety in a musculoskeletal setting.
Subsequent clinical trials demonstrated that ixekizumab significantly improved disease activity, spinal pain, and physical function, with higher ASAS 20 and ASAS 40 response rates compared with placebo. These benefits were observed in both biologic-naïve patients and those previously treated with other biologics, supporting its use as an important alternative option in AS management.
The approval of ixekizumab for ankylosing spondylitis therefore represents not an extension by assumption, but a data-driven expansion based on disease-specific evidence.
👉 Together, these indications reflect the evolving role of ixekizumab across immune-mediated inflammatory diseases, guided by both shared inflammatory mechanisms and condition-specific clinical evidence.
How Is Ixekizumab Different from Steroids or Traditional Immunosuppressants?
This is one of the most common questions among patients. Although these therapies all aim to control inflammation, their key difference lies in how precisely they act on the immune system.
| Therapy Type | Immune Targeting | Typical Role |
| Corticosteroids | Broad, non-selective | Short-term symptom control |
| Conventional immunosuppressants | Broad immune suppression | Disease control with careful monitoring |
| Ixekizumab (biologic therapy) | Highly selective (IL-17A) | Long-term, targeted disease management |
How These Differences Affect Treatment Decisions
- Corticosteroids
Because corticosteroids act across multiple immune and metabolic pathways at the same time, they can quickly suppress inflammation regardless of its source.
This explains their rapid clinical effect—but also why prolonged exposure often leads to systemic complications, limiting their role to short-term or acute disease control.
- Conventional immunosuppressants
By interfering with immune cell proliferation and function, conventional immunosuppressants gradually reduce inflammatory activity.
Their broader mechanism accounts for both their slower onset and the need for ongoing laboratory monitoring, as normal immune and hematologic processes may also be affected.
- Ixekizumab (targeted biologic therapy)
Ixekizumab was designed based on the identification of IL-17A as a central driver of inflammation in specific immune-mediated diseases.
By interrupting this single pathway, it addresses the underlying disease mechanism more directly—explaining its ability to deliver sustained disease control without generalized immune suppression when used under appropriate medical supervision.
👉 Rather than choosing between “strong” or “weak” drugs, modern treatment decisions focus on mechanism-based precision. Ixekizumab reflects this shift—offering targeted efficacy designed around disease biology, not generalized immune suppression.
How Is Ixekizumab Used and What Should Be Considered?
👉 Ixekizumab is a prescription biologic medication and should always be used under the guidance of a qualified healthcare professional.
It is administered by subcutaneous injection and, as a biologic therapy, is not taken orally. Proper handling and storage are important to maintain product stability and therapeutic effectiveness.
Ixekizumab is intended for the long-term management of chronic immune-mediated inflammatory diseases, rather than for rapid, short-term symptom suppression. Treatment decisions are individualized and take into account disease severity, previous treatment history, and overall clinical assessment.
In many cases, ixekizumab is considered when conventional therapies fail to provide adequate disease control or are not well tolerated.
- Because ixekizumab works by modulating immune signaling pathways, patients may be assessed for potential infection risks and other immune-related considerations before starting therapy.
- Including an increased risk of infections and other immune-related adverse events, which require appropriate clinical assessment and monitoring.
- During treatment, regular medical follow-up helps evaluate clinical response, monitor safety, and support appropriate long-term disease management.
❗ Patients should not initiate, discontinue, or adjust treatment on their own, as ongoing professional supervision is essential to ensure both effectiveness and safety.

Taltz (Ixekizumab) – Plaque Psoriasis | HongKong DengYue Medicine
- Generic Name/Brand Name: Ixekizumab/Taltz
- Indications: Plaque Psoriasis
- Dosage Form: Injection
- Specification: 80mg/ml x 1 syringe
Conclusion
Understanding what is Taltz ixekizumab used for helps clarify why ixekizumab has become an important option in the treatment of chronic inflammatory diseases.
By specifically targeting IL-17A, it controls inflammation at its source in conditions such as plaque psoriasis and ankylosing spondylitis, rather than broadly suppressing the immune system.
As precision medicine continues to evolve, accurate drug information—supported by healthcare professionals and partners such as Dengyue Medicine—plays a key role in promoting safe and appropriate treatment decisions.
FAQ about What Is Taltz Ixekizumab Used For
How quickly does Taltz work for psoriasis?
Treatment with Taltz starts to improve symptoms in patients with psoriasis within about 1-2 weeks, according to clinical trial results.
What does Taltz do to your immune system?
TALTZ may lower the ability of your immune system to fight infections and may increase your risk of infections.
What should I avoid while using Taltz?
Taltz interactions with other medications:
1. Tofacitinib (Xeljanz, Xeljanz XR) can increase the risk of side effects of Taltz and tofacitinib.
2. Abatacept (Orencia) can increase the risk of side effects of Taltz and abatacept.
What foods make psoriasis worse?
Nightshade plants include tomatoes, peppers, eggplant and white potatoes.



