What Is Ponatinib Used For? Targeting T315I-Mutant CML and Ph+ ALL
In the treatment of leukemia, not all patients respond equally to standard therapies. Drug resistance and genetic mutations can significantly reduce the effectiveness of commonly used treatments, creating an urgent need for more advanced targeted options.
At the same time, as innovative medicines continue to emerge, the field of hematologic oncology is evolving rapidly. As an integral part of the pharmaceutical supply chain, we focus not only on medicine accessibility but also on understanding the clinical value behind these therapies in order to better serve healthcare providers.
Against this evolving clinical landscape, ponatinib has emerged as an important targeted therapy. What is ponatinib used for? This article explores its mechanism of action, key clinical characteristics, and the role it plays in addressing treatment-resistant leukemia.
Clinical Background and Unmet Medical Needs in the Era of TKI Resistance
Chronic myeloid leukemia (CML) and Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) are hematologic malignancies driven by the BCR-ABL1 fusion gene, which results in constitutive activation of tyrosine kinase signaling and uncontrolled leukemic cell proliferation.
👉 The introduction of tyrosine kinase inhibitors (TKIs) has transformed the treatment landscape, significantly improving survival and converting these diseases into more manageable chronic conditions for many patients.
However, in the era of widespread TKI use, treatment resistance and disease progression remain major clinical challenges. Although first- and second-generation TKIs achieve durable responses in a substantial proportion of patients, a subset develops resistance or intolerance over time, limiting long-term disease control.
Resistance may arise from multiple mechanisms, most notably BCR-ABL1 kinase domain mutations.
Among these, the T315I gatekeeper mutation represents one of the most difficult obstacles in clinical practice, as it prevents effective binding of most earlier-generation TKIs. Patients with T315I mutations or resistance to multiple TKIs often face limited therapeutic options and poorer clinical outcomes.
Consequently, there remains a clear and ongoing unmet medical need for potent, mutation-resistant therapies capable of addressing TKI-resistant disease and improving outcomes in patients with advanced or refractory CML and Ph+ ALL.
What Is Ponatinib and How Does It Work?
Ponatinib tablets were developed as a precision-targeted therapy to address the challenge of resistance to prior tyrosine kinase inhibitor (TKI) treatment.
It is the first third-generation TKI worldwide—and currently the only one approved in China—indicated for patients with chronic myeloid leukemia (CML) across all disease phases (chronic phase, accelerated phase, and blast phase) and Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) who are resistant or intolerant to previous TKIs.
✨ The core value of ponatinib lies in its unique molecular design, which directly tackles two of the most difficult clinical challenges in this setting: resistance driven by the T315I mutation and leukemic infiltration of the central nervous system (CNS).
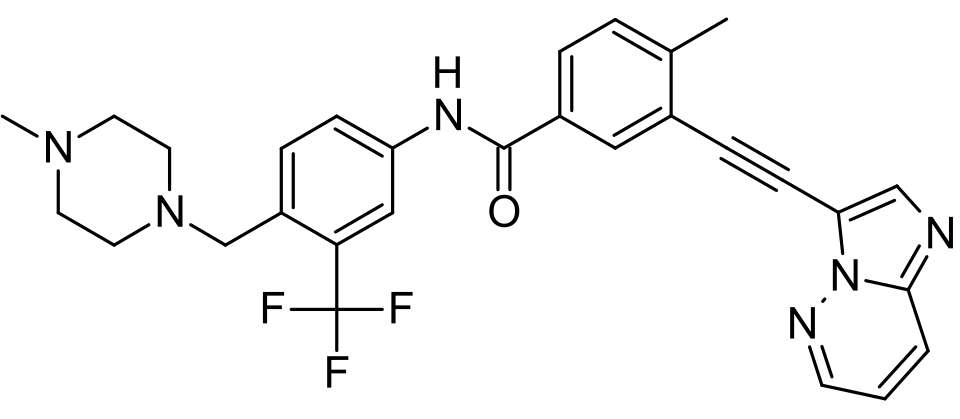
1️⃣ Structural Innovation: Overcoming the Steric Hindrance of the T315I Mutation
At its core, drug resistance arises from structural alterations in the target protein, namely the BCR-ABL1 kinase.
Among all resistance mutations, the T315I point mutation is one of the most challenging. This mutation introduces a bulky isoleucine residue at a critical position within the ATP-binding domain of the kinase, creating significant steric hindrance that physically blocks the binding of most first- and second-generation TKIs, ultimately leading to treatment failure.
The success of ponatinib stems from a structure-based, rational drug design strategy.
🧬 Its molecular backbone incorporates a distinctive carbon–carbon triple bond linker. This rigid structural element, defined by precise bond length and geometry, allows the molecule to adopt an optimized conformation that accommodates the altered kinase domain.
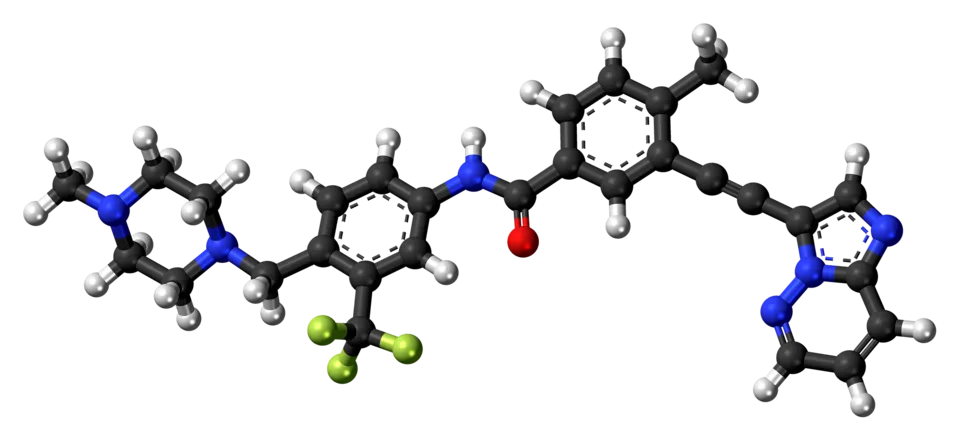
By effectively avoiding steric clashes with the mutant residue, ponatinib can stably occupy the ATP-binding pocket and potently inhibit the activity of more than 40 BCR-ABL1 resistance mutations, including T315I.
2️⃣ Multi-Target Inhibition: Broader Coverage of Oncogenic Signaling Pathways
Beyond its potent inhibition of BCR-ABL1, ponatinib was intentionally designed as a multi-target tyrosine kinase inhibitor.
It also strongly inhibits several other kinases that are closely associated with leukemic cell growth, survival, and disease progression, including:
- VEGFR, PDGFR, and FGFR, which play important roles in tumor angiogenesis and regulation of the tumor microenvironment
- SRC family kinases, which are involved in signaling pathways governing cell proliferation and survival
This broad target profile offers additional potential clinical benefits.
In addition to directly suppressing the primary leukemogenic signaling axis, ponatinib may also reduce or delay the emergence of bypass resistance driven by alternative pathway activation, providing a more comprehensive therapeutic strategy in complex, treatment-resistant disease settings.
3️⃣ Optimized Physicochemical Properties: Effective Penetration of the Central Nervous System
Central nervous system (CNS) infiltration by leukemic cells is a major cause of treatment failure and relapse. However, most TKIs have limited ability to cross the blood–brain barrier, making CNS disease difficult to control.
During its development, ponatinib was optimized in terms of key physicochemical properties such as lipophilicity and molecular weight, resulting in superior blood–brain barrier penetration compared with other TKIs in its class.
As a result, ponatinib can achieve and maintain therapeutically relevant concentrations in the cerebrospinal fluid. Clinically, this enables not only the treatment of established CNS leukemia, such as leptomeningeal disease, but also offers a preventive benefit by reducing the risk of CNS relapse in high-risk patients.
👉 This supports an integrated “systemic plus CNS” approach to deep and durable disease control.
In summary, through its structural innovation that overcomes key resistance mutations, its broad inhibition of multiple oncogenic signaling pathways, and its optimized tissue distribution—particularly within the CNS—ponatinib has established a distinct therapeutic advantage in the management of refractory CML and Ph+ ALL.
It represents an effective and critically important treatment option for clinicians and patients facing limited alternatives.
What Is Ponatinib Used For? Clinical Applications and Comparative Value of Ponatinib
The clinical value of Ponatinib (Iclusig) lies in its clearly demonstrated advantages across key treatment scenarios. According to data reviewed during China’s national reimbursement evaluation, comparative evidence between ponatinib and olverembatinib—both third-generation TKIs—provides a direct basis for defining its core clinical applications.
Core Indication: A Preferred Option for Resistant CP-CML Patients Seeking Deep Remission
For patients with chronic-phase chronic myeloid leukemia (CP-CML) who are resistant or intolerant to prior TKIs, Iclusig represents an important option for achieving deep remission.
👉 In key efficacy comparisons with olverembatinib, ponatinib demonstrated higher response rates across multiple clinical endpoints (detailed comparative data are presented in Section IV).
These findings suggest that, in this core setting, ponatinib offers clinicians a treatment strategy aimed at achieving deeper and more durable disease control, supporting more ambitious therapeutic goals for resistant CP-CML.
Key Differentiated Scenarios: CNS Involvement and T315I-Mutated Advanced Disease
Ponatinib holds a clearly defined clinical role—grounded in its unique pharmacologic properties—in two high-unmet-need scenarios.
- Scenario 1: Prevention and Treatment of Central Nervous System (CNS) Involvement
Iclusig demonstrates the strong blood–brain barrier penetration among currently available TKIs.
Accordingly, for CML or Ph+ ALL patients with high risk of CNS relapse, as well as those with confirmed CNS involvement, ponatinib plays a critical role in achieving effective, synchronized control of both systemic and CNS disease.
This characteristic represents one of its most important differentiating features compared with other TKIs.
- Scenario 2: Treatment of T315I-Mutated Blast-Phase CML and Ph+ ALL
Iclusig’s unique molecular structure enables potent inhibition of the T315I mutation, a major driver of resistance to earlier-generation TKIs.
This capability allows ponatinib to fill a previously unmet treatment gap in China. For patients with genetically confirmed T315I mutation whose disease has progressed to accelerated phase or blast phase CML, or who have Ph+ ALL, ponatinib is currently a guideline-recommended standard treatment option with domestic accessibility.
The core clinical positioning of ponatinib focuses on three key scenarios:
achieving deep remission in resistant CP-CML, preventing and treating CNS involvement, and managing T315I-mutated advanced-phase CML and Ph+ ALL.
Its specific advantages in each scenario will be further clarified through a systematic comparison with olverembatinib in the following section.
In-Depth Comparison: Differentiated Advantages of Ponatinib vs. Olverembatinib
Although both are classified as third-generation TKIs, ponatinib and olverembatinib (Nailike) differ significantly across several key attributes, and these differences have direct implications for clinical decision-making.
The following systematic comparison—based on available efficacy data and pharmacologic characteristics—outlines the core rationale for selecting ponatinib in specific clinical scenarios.
Depth of Efficacy: Higher Cytogenetic and Molecular Response Rates
For patients with TKI-resistant chronic-phase CML (CP-CML) whose treatment goal is to achieve deep remission, ponatinib has demonstrated more favorable efficacy outcomes. Higher response rates are associated with improved quality of disease control and a lower short-term risk of disease progression.
| Key Efficacy Endpoint | Ponatinib | Olverembatinib | Clinical Significance |
| Major cytogenetic response (MCyR) | 60% | 47.7% | A foundational indicator of chromosomal-level disease control |
| Complete cytogenetic response (CCyR) | 54% | 36.4% | Reflects deeper cytogenetic remission and is associated with better long-term outcomes |
| Major molecular response (MMR) | 40% | 27.3% | Indicates deep molecular remission and is a prerequisite for treatment-free remission considerations |
Safety Profiles: Differentiated Adverse Event Management Priorities
The two agents exhibit distinct safety profiles, which influence treatment tolerability and monitoring strategies.
- Hematologic and Hepatic Toxicity:
- Ponatinib has shown relatively lower rates of grade 3–4 thrombocytopenia, anemia, and transaminase elevation, potentially making it more suitable for patients with compromised baseline blood counts or hepatic function.
- Cardiovascular Risk: Both agents require attention to arterial occlusive events.
- For ponatinib, extensive global experience has established a structured “A-to-E” comprehensive risk management strategy—ranging from baseline assessment to lifestyle and exercise management—which has demonstrated controllable risk in real-world settings.
- In clinical practice, baseline cardiovascular risk assessment and individualized monitoring plans are essential for all patients.
Pharmacologic Characteristics: Determinants of Clinical Applicability
Pharmacologic properties represent the most fundamental distinction between the two agents and directly define their respective clinical roles.
- Blood–Brain Barrier Penetration: Ponatinib demonstrates the strongest central nervous system (CNS) penetration among currently available TKIs.
👉 Consequently, for patients with confirmed CNS involvement or a high risk of CNS relapse, ponatinib has demonstrated pharmacologic properties and clinical experience suggestive of effective systemic and CNS disease control. These features differentiate it from other third-generation TKIs such as olverembatinib, for which limited CNS-specific evidence is currently available.
- Breadth of Target Inhibition: Ponatinib functions as a multi-kinase inhibitor, with activity against targets such as VEGFR and FGFR, potentially enabling broader suppression of oncogenic signaling pathways.
👉 In contrast, olverembatinib has a more selective target profile. These differences in kinase coverage may influence each drug’s ability to overcome specific resistance mechanisms.
The choice of ponatinib over other third-generation TKIs is based on precise alignment with specific clinical needs.
Conclusion
Iclusig® (ponatinib) represents a key therapeutic advance for patients with resistant or high-risk CML and Ph+ ALL. As a third-generation BCR-ABL inhibitor, it was specifically developed to overcome T315I-mediated resistance and to improve disease control, including protection against central nervous system involvement.
From a clinical perspective, what ponatinib is used for is no longer limited to late-line salvage therapy, but rather for precision treatment of clearly defined, high-unmet-need patient populations. With its validated mechanism of action and increasing accessibility, the ponatinib tablet delivers both clinical and real-world value.
As a responsible global drug wholesale distributor, DengYueMedicine are fully prepared to ensure the stable supply and professional service of this important medicine.
FAQ about What Is Ponatinib Used For
What type of drug is ponatinib?
Ponatinib is in a class of medications called kinase inhibitors.
What should I avoid while taking ponatinib?
Notes for Consumers: Avoid grapefruit or grapefruit juice while taking ponatinib.
What are the side effects of ponatinib?
1. Bladder pain.
2. Bleeding gums.
3. Bloating or swelling of the face, arms, hands, lower legs, or feet.
4. Burning, numbness, tingling, or painful sensations.
5. Chest pain.
6. Cloudy urine.
7. Confusion.
8. Constipation.
What is the success rate of ponatinib?
Overall and progression-free survival were 85.8% and 83.8% in CML patients after 48 months of treatment, and 82.5% and 54.2% in Ph+ ALL patients after 30 months of treatment.