
What Class of Drug Is Penpulimab? How This Fc-Engineered PD-1 Monoclonal Antibody Restores Anti-Tumor Immunity?
In recent years, immune checkpoint inhibitors have revolutionized the field of anti-tumor treatment, among which PD-1 (programmed death protein 1) monoclonal antibodies have become a core therapeutic option for multiple advanced tumors due to their unique mechanism of action and significant clinical efficacy.
Penpulimab, with its brand name Anike®, as a novel PD-1 inhibitor with independent innovation in China, has attracted widespread attention in the medical community due to its differentiated molecular design and excellent clinical performance.
As a professional pharmaceutical supply service provider, DengYue Meedicine closely follows the global development trends of innovative oncology therapies and is committed to improving access to high-quality anti-tumor medicines.
👉 Against this backdrop, this article will focus on the core question, “What class of drug is Penpulimab?” and systematically elaborate on its basic identity, anti-tumor mechanism, unique advantages compared with similar drugs, and clinical value to help readers fully understand this innovative anti-tumor drug.
What is Anike?
To answer the question “What class of drug is Penpulimab?” clearly, we first clarify its basic identity: Penpulimab Injection, whose brand name is Anike®, is a novel PD-1 immune checkpoint inhibitor developed independently in China by Akeso Biopharma in collaboration with Chia Tai Tianqing Pharmaceutical Group.
Specifically, it belongs to the class of humanized IgG1 monoclonal antibodies targeting programmed death protein 1 (PD-1, also known as PDCD1 or CD279), a key immune regulatory molecule in the human body.
As a prescription anti-tumor drug, Penpulimab (Anike®) has been continuously approved for new indications at home and abroad since its launch, covering multiple major tumor types. Its approval timeline and key indications are as follows:
- August 2021: Approved by China’s NMPA for the treatment of relapsed or refractory classical Hodgkin lymphoma (initial indication);
- Subsequently, NMPA expanded its indications to include locally advanced/metastatic squamous non-small cell lung cancer, recurrent/metastatic nasopharyngeal carcinoma, and advanced hepatocellular carcinoma;
- April 2025: Approved by the U.S. FDA for nasopharyngeal carcinoma-related indications, further expanding its global clinical application scope.
In summary, Penpulimab (Anike®) is clearly classified as a PD-1 inhibitor in the field of anti-tumor drugs, and its unique molecular design (IgG1 subtype with Fc segment modification) distinguishes it from other drugs in the same class, laying a foundation for its advantages in efficacy and safety.
How Does Anike (Penpulimab) Work?
The core anti-tumor effect of Penpulimab (Anike®) relies on its specific regulation of the immune checkpoint pathway, and its Fc-engineered design further optimizes this mechanism to achieve more effective and safer anti-tumor immunity restoration, which can be divided into two key links:
First, blocking the PD-1/PD-L1 immune escape pathway of tumor cells.
Under normal physiological conditions, PD-1, a transmembrane protein expressed on the surface of T cells (a key component of the body’s immune system), acts as an “immune brake”.
👉 When PD-1 binds to its ligands PD-L1 (programmed death ligand 1) or PD-L2 (programmed death ligand 2) expressed on normal cells, it can inhibit the activation and effector function of T cells, preventing the immune system from mistakenly attacking normal tissues.
However, many tumor cells will abnormally overexpress PD-L1, which binds to PD-1 on T cells to “deceive” the immune system, making T cells lose their ability to recognize and kill tumor cells, thereby achieving immune escape and continuous proliferation of tumor cells.
Penpulimab specifically binds to PD-1 on the surface of T cells with high affinity, occupying the binding site of PD-1 and blocking its interaction with PD-L1/PD-L2 ligands on tumor cells.
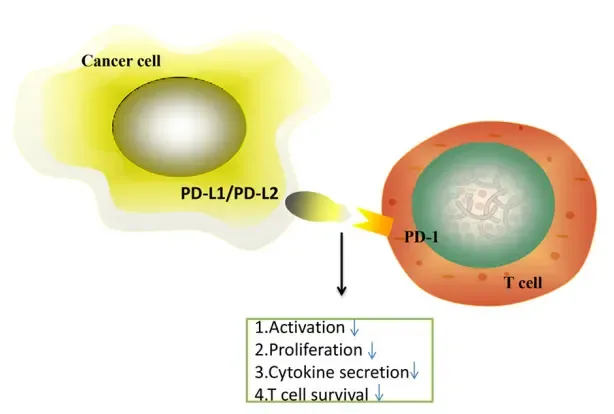
This process is equivalent to “releasing the brake” of the immune system, enabling T cells to regain their activation ability, recognize tumor cells accurately, and initiate a specific T cell-mediated immune response to attack and eliminate tumor cells, thereby achieving the purpose of anti-tumor treatment.
Second, the Fc segment modification enhances efficacy and reduces adverse reactions.
👉 Different from most PD-1 inhibitors of the IgG4 subtype, Penpulimab adopts an IgG1 subtype and undergoes targeted modification of the Fc segment, completely eliminating its ability to bind to Fcγ receptors (FcγR).
This modification can avoid antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP) effects, reducing the consumption of effector T cells mediated by macrophages and the release of pro-inflammatory cytokines such as IL-6 and IL-8, thereby not only enhancing the persistence of T cell anti-tumor activity but also fundamentally reducing the occurrence of immune-related adverse reactions (IRAEs).
In addition, studies have shown that Penpulimab binds to the N-glycosylation site (N58 residue) of the PD-1 receptor, with a slower dissociation rate from PD-1 compared to other PD-1 inhibitors, which can achieve higher receptor occupancy and further enhance T cell activity, making the anti-tumor effect more durable and powerful.
What Makes Anike (Penpulimab) Different from Other Drugs of the Same Class? (Advantages)
As a new generation of PD-1 inhibitors, Penpulimab (Anike®) has formed obvious differentiated advantages compared with other PD-1 monoclonal antibodies currently on the market, mainly reflected in three core aspects, all closely related to its unique molecular design and clinical performance:
✨ The first and most core advantage is the unique molecular design of IgG1 subtype + Fc segment modification.
There are obvious differences between Penpulimab and most PD-1 inhibitors of the IgG4 subtype currently used in clinical practice, as shown in the following table:
| Characteristics | Penpulimab (IgG1 Subtype + Fc Modification) | Most Other PD-1 Inhibitors (IgG4 Subtype) |
|---|---|---|
| Structural Stability | More stable, no aggregation problem | Poor stability, easy to aggregate |
| Binding to Anti-Tumor IgG1 | No binding, no reduction in anti-tumor activity | May bind, reducing anti-tumor activity or promoting tumor immune escape |
| Binding to Fcγ Receptors | Completely eliminated (after Fc modification) | May bind, triggering unnecessary immune activation |
| ADCC/CDC/ADCP Effects | Avoided, reducing T cell consumption and IRAEs | May occur, increasing related adverse reactions |
✨ The second advantage is superior clinical efficacy in multiple tumor types.
Compared with other PD-1 inhibitors, Penpulimab has shown leading performance in key clinical indicators of multiple approved indications, as detailed below:
| Tumor Type | Key Clinical Indicators | Performance of Penpulimab |
|---|---|---|
| Squamous Non-Small Cell Lung Cancer (First-Line Treatment) | 30-month Overall Survival Rate (OS Rate) | Exceeds 50% (the world’s first PD-1 monoclonal antibody to achieve this) |
| Metastatic Nasopharyngeal Carcinoma (Third-Line Treatment) | Median Overall Survival (OS) | Reaches 22.8 months (ranking first among similar drugs) |
| Metastatic Nasopharyngeal Carcinoma (First-Line Treatment) | Median Progression-Free Survival (PFS); 12-month PFS Rate | Median PFS reaches 9.6 months; 12-month PFS rate increases from 11% to 31% |
| Relapsed/Refractory Classical Hodgkin Lymphoma | Objective Response Rate (ORR); Complete Response Rate (CR); 18-month OS Rate | ORR: 89.4%; CR: 47.1%; 18-month OS Rate: 100% |
✨ The third advantage is better safety and tolerability.
Safety is the key to long-term tumor treatment, and Penpulimab has obvious advantages in this regard due to its Fc segment modification, mainly reflected in the following aspects:
- Low incidence of severe immune-related adverse reactions (IRAEs): Clinical data show that the incidence of grade 3 or above severe IRAEs is only 3.2%-4.3%, far lower than that of similar drugs;
- No severe adverse reactions of grade 4 or 5 have been observed, which is safer for long-term treatment;
- Mild and tolerable common adverse reactions: Mainly mild to moderate reactions such as fatigue, pruritus, diarrhea, and rash;
- Good tolerance when combined with chemotherapy: Adverse reactions are mainly mild gastrointestinal reactions, no frequent dose adjustment is required, and patients can stably complete the treatment cycle, reducing treatment interruption caused by adverse reactions.
Conclusion
To answer the core question “what class of drug is Penpulimab” comprehensively: Penpulimab (brand name: Anike®) is a PD-1 immune checkpoint inhibitor belonging to the class of humanized IgG1 monoclonal antibodies, which targets the PD-1 receptor to exert anti-tumor effects and is mainly used for the treatment of multiple advanced malignant tumors such as classical Hodgkin lymphoma, squamous non-small cell lung cancer, nasopharyngeal carcinoma, and hepatocellular carcinoma.
Different from other PD-1 inhibitors of the same class, Penpulimab achieves a breakthrough in “efficacy and safety” through its unique molecular design of IgG1 subtype + Fc segment modification:
- On the one hand, it blocks the PD-1/PD-L1 pathway to awaken the body’s own immune system, enabling T cells to effectively recognize and kill tumor cells;
- On the other hand, it avoids unnecessary immune activation through Fc segment modification, reduces the consumption of effector T cells and the release of pro-inflammatory cytokines, and achieves a balance between potent anti-tumor effect and mild tolerance.
As a representative of innovative PD-1 inhibitors with independent intellectual property rights in China, Penpulimab not only enriches the clinical treatment options for tumors but also provides a new direction for the research and development of PD-1 inhibitors with its differentiated advantages, highlighting the great progress of China’s innovative anti-tumor drugs in the global field.
FAQ about What Class of Drug Is Penpulimab?
What class of drug is penpulimab?
Penpulimab-kcqx is in a class of medications called monoclonal antibodies.
Is penpulimab approved by the FDA?
On April 23, 2025, the Food and Drug Administration approved penpulimab-kcqx (Akeso Biopharma Co., Ltd.) with cisplatin or carboplatin and gemcitabine for the first-line treatment of adults with recurrent or metastatic non-keratinizing nasopharyngeal carcinoma (NPC).
What is the indication of penpulimab?
Penpulimab-kcqx, as a single agent, is indicated for the treatment of adults with metastatic non- keratinizing NPC and disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy.
Who makes penpulimab?
It is developed by Akeso.



