Combating the “Silent Killer”: How Atrasentan is Redefining Primary IgA Nephropathy Treatment and Altering the Course to Kidney Failure
🤔 In China, what is the most common primary glomerulonephritis leading to kidney failure in young and middle-aged adults?
The answer is primary IgA nephropathy.
👉 This disease has an insidious onset, often presenting initially only as microscopic hematuria or mild proteinuria without noticeable discomfort for the patient.
👉 Yet, it can silently erode kidney function, pushing 30–40% of patients toward end-stage renal disease (kidney failure) within 10–20 years.
For a long time, traditional primary IgA nephropathy treatment regimens have shown a clear “efficacy ceiling” for high-risk patients with persistently elevated proteinuria.
When optimized foundational therapy still proves insufficient to control disease progression, doctors and patients often find themselves in a dilemma with limited options. However, medical breakthroughs often emerge from silence.
💊 With a deeper understanding of the disease’s molecular mechanisms, a novel targeted therapy—Atrasentan Hydrochloride Tablets—has been approved in China, offering a new tool to delay kidney failure and potentially alter the disease course for specific high-risk patients.

Next, Hong Kong DengYue Medicine will guide you through a systematic understanding of primary IgA nephropathy and provide an in-depth analysis of the scientific rationale and clinical application behind this innovative drug
Helping you understand why it is regarded as a significant breakthrough in the treatment landscape.
Understanding Primary IgA Nephropathy—The Risk Lies in the “Proteinuria” Indicator
Primary IgA nephropathy is not merely a case of “kidney inflammation.”
At its core, it stems from an immune system dysfunction that produces abnormal antibodies (IgA), which deposit in the kidneys’ filtration units (glomeruli).
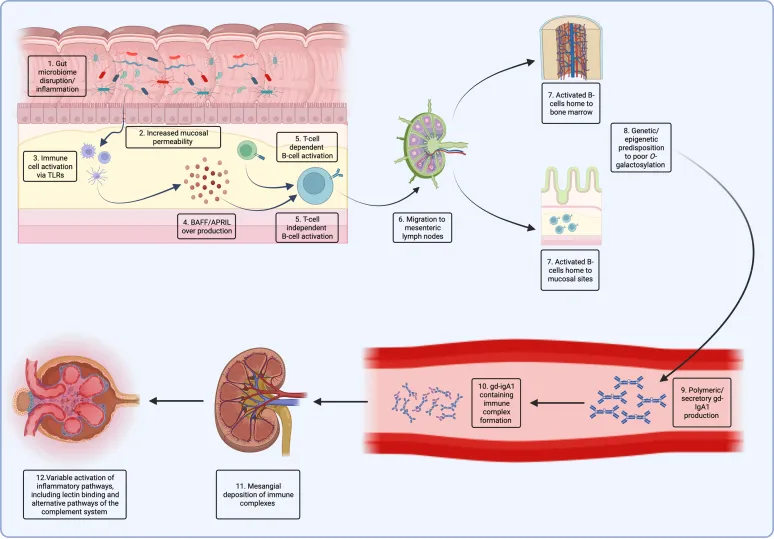
This triggers persistent immune damage and inflammatory responses, akin to debris accumulating on a filter, ultimately leading to the hardening and deterioration of the filtration system.
🛏️ Clinically, the gold standard for assessing disease severity and prognosis is the 24-hour urinary protein quantification.
⚠️ Particularly when urinary protein persistently measures ≥1.0 grams per day, it is regarded as a clear “high-risk” signal.
Significant proteinuria not only reflects severe damage to the glomerular filtration barrier but also further harms the renal tubules, accelerating kidney fibrosis and atrophy.
Therefore, reducing and controlling proteinuria is one of the most critical therapeutic goals for slowing the progression of IgA nephropathy.
The “Efficacy Ceiling” of Traditional Therapies and Unmet Clinical Needs
Over the past two decades, the cornerstone of foundational treatment for IgA nephropathy has been RAS inhibitors (i.e., ACE inhibitors or ARBs).
✅ These drugs reduce intraglomerular pressure and decrease proteinuria and have been proven to delay the decline of kidney function, making them the undisputed first-line choice.
😐 However, a distinct “efficacy ceiling” is observed in clinical practice: even when titrated to the maximum tolerated dose, up to 30–40% of high-risk patients still fail to achieve safe proteinuria levels (<0.5–1.0 g/day), and their kidney function continues to decline gradually.
For these patients, clinicians might previously have considered adding corticosteroids or other immunosuppressants, but these agents carry substantial risks of side effects such as infections and metabolic disturbances, and their efficacy is not consistently reliable for all patients.
Therefore, there is an urgent clinical need for a new generation of therapies for high-risk IgA nephropathy patients.
This pressing demand has directly driven research into drugs targeting the disease’s core pathogenic pathways, paving the way for novel treatment paradigms in primary IgA nephropathy.
✨ This evolving landscape includes new insights and emerging therapies, among which the introduction of Atrasentan Hydrochloride Tablets marks a significant advancement in primary IgA nephropathy treatment.
A Breakthrough in Mechanism—The “Precision Strike” of Atrasentan
Scientific research has revealed that in the progression of IgA nephropathy, a molecular pathway known as the “endothelin-1 pathway” plays a key “antagonist” role.
Endothelin-1 is a potent vasoconstrictor that is overexpressed in damaged kidneys, leading to three major detrimental outcomes:
- Sustained constriction of blood vessels within the glomeruli, increasing pressure;
- Promotion of inflammatory responses;
- Direct stimulation of renal fibroblasts, resulting in irreversible renal fibrosis (scarring).
👉 The innovativeness of Atrasentan Hydrochloride Tablets lies precisely in targeting this critical pathway.
✨ As a highly selective endothelin-A receptor antagonist, it can be understood as a precisely designed “molecular key” that accurately blocks the “keyhole” (the A receptor) through which endothelin-1 exerts its harmful effects, thereby preventing all its detrimental signals.
This “precision strike” delivers multiple benefits:
- Dilates renal blood vessels, reducing intraglomerular high pressure.
- Exerts anti-inflammatory and anti-fibrotic effects, structurally protecting the kidneys.
- Significantly reduces proteinuria, interrupting the vicious cycle of “proteinuria–kidney damage.”
This mechanism of action, targeting a specific single pathway, represents a conceptual leap in the treatment of primary immunoglobulin A (IgA) nephropathy—from “broad immunosuppression” to “precise blockade of the core pathogenic pathway.”
Such progress exemplifies the novel treatment paradigms and new insights and emerging therapies in primary IgA nephropathy treatment.
Now, the question arises: What concrete and significant changes has this theoretical breakthrough brought to patients in real-world clinical applications?
Clear Clinical Value and Groundbreaking Efficacy Evidence
Supported by robust evidence from the global Phase III PROTECT study, Atrasentan Hydrochloride Tablets have been approved in China for the treatment of adult patients with primary IgA nephropathy and urinary protein ≥1.0 g/day.
The study results have garnered significant attention in the medical community, offering new therapeutic hope for high-risk IgA nephropathy patients.
Detailed Analysis of Key Research Data:
- Significant Reduction in Proteinuria: At 36 weeks of treatment, compared to the placebo group, the atrasentan 0.75 mg/day dose group achieved an average reduction of 47.1% in proteinuria levels.
✅ More importantly, over 33% of patients achieved the treatment goal of proteinuria ≤1 g/day, a proportion significantly higher than that in the placebo group (approximately 16%).
- Stabilization of Kidney Function: After 136 weeks (approximately 2.5 years) of long-term follow-up, the study found that the annual rate of decline in estimated glomerular filtration rate (eGFR) was significantly slower in the atrasentan treatment group.
✅ Specifically, eGFR in the treatment group declined by an average of approximately -4.17 mL/min/1.73 m² per year, compared to -5.28 mL/min/1.73 m² in the placebo group, demonstrating a relative protective effect of about 21%.
Delay in Disease Progression: Interim analysis of the study showed that the proportion of patients experiencing a composite kidney function endpoint event (including a ≥40% decline in eGFR, progression to end-stage kidney disease, or kidney-related death) was significantly lower in the atrasentan treatment group.
✅ The hazard ratio was approximately 0.65, indicating a 35% reduction in the risk of progression.
This means that for eligible high-risk patients, this drug provides a treatment option that goes beyond traditional therapies, targeting “hard endpoints” (i.e., delaying the onset of kidney failure).
✨ It is typically not used as monotherapy but is combined with optimized RAS inhibitor therapy, forming a reinforced “dual-pressure reduction, dual-protection” strategy to collectively build a stronger protective network for the kidneys.
Based on rigorous clinical research data, atrasentan has demonstrated clear kidney-protective value for high-risk IgA nephropathy patients.
🤔 Now, with a clear understanding of its clinical benefits, how should eligible patients scientifically initiate treatment? How can they maximize therapeutic outcomes through collaboration with their physicians?
Patient Action Guide—The Scientific Path from Awareness to Decision-Making
If you or a family member has been diagnosed with IgA nephropathy and is considering evaluating new treatment options, you can follow this pathway:
1️⃣ Complete a Precise Assessment
- Confirm the Diagnosis: Kidney biopsy remains the gold standard for diagnosing IgA nephropathy.
- Quantify the Risk: Accurately measure 24-hour urinary protein quantification and estimated glomerular filtration rate (eGFR). This is the foundation for determining whether you belong to the “high-risk” category and meet the indications for medication, such as Atrasentan Hydrochloride Tablets.
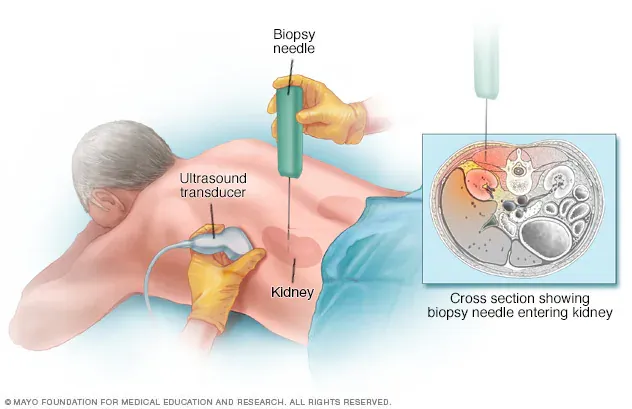
2️⃣ Engage in In-Depth Communication with a Nephrology Specialist
Bring your examination reports and discuss the following key points with your doctor:
- “Is my urinary protein level persistently ≥1.0 g/day?”
- “Based on my overall health profile, what are the potential benefits and risks of using Atrasentan Hydrochloride Tablets?”
- “If prescribed, what is the specific plan for monitoring liver function? How should potential side effects be managed?”
3️⃣ Reach Consensus, Standardize Treatment, and Ensure Follow-Up
- Once the decision is made to initiate treatment, strictly adhere to the prescribed medication regimen. Never adjust the dosage or discontinue the medication without medical guidance.
- Faithfully attend all scheduled follow-up appointments, particularly for monitoring liver function and anemia indicators. This is the core safeguard for treatment safety.
- Maintain open communication with your healthcare team to establish a solid “treatment alliance.”
Acquiring knowledge enables wiser actions. On the long journey of combating disease, scientific understanding, evidence-based decision-making, and a trusting doctor-patient partnership form a more solid foundation than any single medication.
Conclusion
The treatment of primary IgA nephropathy has entered a new era of targeted therapy. The introduction of Atrasentan Hydrochloride Tablets represents more than just the launch of a new medication
It serves as a clear signal that our approach to managing high-risk patients is shifting from a relatively passive strategy of “delaying” decline to a more proactive one of “reducing risk.”
👉 While not a panacea and bound by specific clinical boundaries, for eligible patients who continue to face the threat of progressive kidney function decline despite traditional therapies, it offers a powerful, clinically validated tool capable of meaningfully altering the disease trajectory.
Hope is born from scientific innovation, and safety is anchored in standardized practice.
At Dengyue Medicine, we believe that for every patient, actively engaging with the latest advancements, partnering closely with specialized healthcare providers, and making individualized, well-informed decisions is the most effective way to embrace the promise of this new era in care.

FAQ about Primary IgA Nephropathy Treatment
What is the gold standard for IgA nephropathy?
IgA nephropathy (IgAN) is the most prevalent primary glomerular disease and has been recognized to carry a poor prognosis.
It is therefore critical to identify the patients that will progress to ESKD and start treatments early.
The current gold standard for diagnosis remains kidney biopsy.
Is Atrasentan approved?
Atrasentan was approved for medical use in the United States in April 2025
What level of IgA is concerning?
High IgA (>4 g/L)
What is the new treatment for IgA nephropathy 2025?
More generally, the 2025 guideline recommends that all patients with IgAN be treated with an optimized maximally tolerated dose of either an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin II receptor blocker (ARB)