The IgA Nephropathy New Treatment Revolution: From Supportive Care to Precision Targeting
IgA nephropathy is the most common primary glomerular disease in China, accounting for approximately 40%–50% of all primary glomerulonephritis cases.
For many years, the treatment of IgA nephropathy has primarily relied on supportive therapies, especially angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs).
These drugs have indeed played important roles in reducing proteinuria and controlling blood pressure.
😐 However, they are fundamentally unable to cure the disease, and approximately 30% of patients respond poorly to these treatments, with kidney function continuing to decline.
The root cause of this dilemma lies in the failure of traditional treatments to address the core pathogenesis of the disease.
However, with advances in medical research, scientists have unveiled the mysteries of IgA nephropathy and have developed a series of innovative drugs based on this understanding.
💊 Amid this shift in treatment paradigms, global drug wholesale distributors like DengYue Medicine are helping to accelerate the clinical availability of these breakthrough therapies through efficient drug accessibility services.
Today, the field of IgA nephropathy new treatment is undergoing a revolutionary transformation—from passive supportive care to proactive precision-targeted intervention.
Understanding the Enemy: The Nature and Progression of IgA Nephropathy
To appreciate the value of these innovative treatments, it is first essential to understand what IgA nephropathy is and how it damages the kidneys.
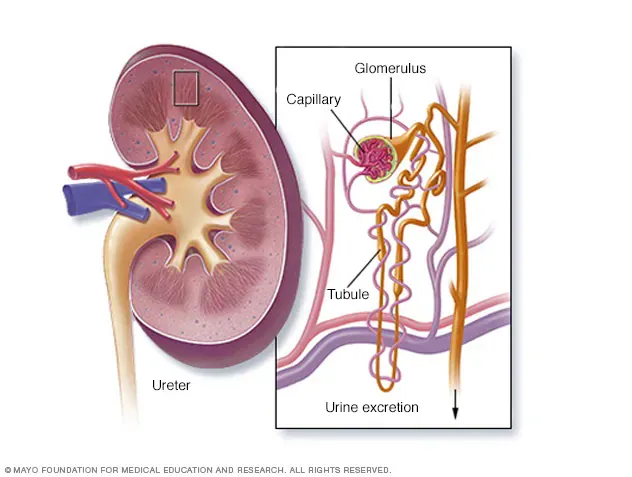
Pathogenesis: The Four-Hit Theory
Modern medicine explains the development of IgA nephropathy through the “Four-Hit” theory:
- The First Hit: The patient’s body produces aberrantly glycosylated IgA1 antibodies (known as Gd-IgA1). This abnormality is primarily linked to genetic factors and dysfunction of the gut mucosal immune system, representing some of the core IgA nephropathy causes.
- The Second Hit: The body produces autoantibodies targeted against these abnormal IgA1 molecules.
- The Third Hit: The abnormal IgA1 and the autoantibodies combine to form immune complexes.
- The Fourth Hit: These complexes deposit in the mesangial areas of the kidney’s glomeruli, activating the complement system and triggering inflammatory responses and fibrotic processes.
In this cascade, the abnormal activation of the complement system (particularly the alternative and lectin pathways) and the overactivity of the endothelin system are key drivers of renal tissue injury and fibrosis.
Clinical Features and Disease Stages
❗ The clinical presentation of IgA nephropathy is diverse and often subtle:
- Classic Presentation: 👨 More common in young males, often presenting with gross hematuria (visible blood in urine) 1-3 days after a respiratory or gastrointestinal infection.
- More Common Presentation: 🩸 Asymptomatic microscopic hematuria accompanied by proteinuria, frequently discovered incidentally during routine physical examinations.
- Diagnostic Gold Standard: 🔬 Renal biopsy, which under pathology reveals predominant IgA immune deposits in the glomerular mesangium.
Risk stratification for disease progression relies on several key indicators, which also help define the IgA nephropathy stages and guide treatment intensity:
- Proteinuria Level: Sustained 24-hour urine protein excretion >1g is a high-risk signal.
- Blood Pressure Control: Hypertension accelerates the deterioration of kidney function.
- Kidney Function Indicators: Serum creatinine levels and estimated Glomerular Filtration Rate (eGFR).
- Pathological Severity: Assessed using the Oxford Classification (MEST-C) to evaluate the degree of kidney tissue damage.
👉 Understanding these IgA nephropathy stages is crucial for determining prognosis and selecting the most appropriate management strategy, moving closer to the goal of making IgA nephropathy curable or at least highly manageable for a greater number of patients.
Based on this profound understanding of the disease’s nature and progression patterns, treatment strategies have undergone a paradigm shift from traditional management to precision intervention.
Therapeutic Evolution: From Traditional Cornerstones to Precision Targeting
In the era of conventional therapy, the medical community’s primary arsenal against IgA nephropathy focused on symptomatic and supportive care.
💊 Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) formed the backbone of treatment, effectively reducing intraglomerular pressure and protein leakage by inhibiting the renin-angiotensin-aldosterone system, thereby slowing the decline of kidney function.
Concurrently, strict comprehensive management—including
- 🥣 A low-salt diet, High-quality low-protein intake
- 📏 Rigorous blood pressure control
- 🦠 Avoidance of nephrotoxic drugs, Infection prevention
—constituted an indispensable foundation of care.
✨ This “supportive treatment” approach has, over the past few decades, helped stabilize the condition of many patients and delayed progression to end-stage renal disease.
🤔 However, the limitations of these traditional methods have become increasingly apparent:
They primarily act on the “downstream” aspects of the disease process, offering symptomatic relief by reducing the kidney’s workload and improving hemodynamics. 🔻
Yet, they fail to address the core of the disease—the fundamental pathogenesis involving abnormal immune responses leading to the production of pathogenic IgA, immune complex deposition, and subsequent complement activation.
Consequently, approximately one-third of patients continue to experience a decline in kidney function despite standardized treatment, ultimately facing dialysis or transplantation.
It is precisely this unmet clinical need that has driven a fundamental shift in therapeutic philosophy.
With deeper insights into key pathological mechanisms such as the “Four-Hit” hypothesis, precision interventions targeting specific critical pathways have become possible.
The generation of IgA nephropathy new treatment strategies no longer aims merely to alleviate symptoms but seeks to interrupt or even reverse the fundamental disease process, thereby genuinely altering the natural history of IgA nephropathy.
This paradigm shift from “broad support” to “precision targeting” marks the official entry of IgA nephropathy management into a new era.
Four Innovative Drugs: New Weapons Rewriting the Disease Course
1. Budesonide Enteric Capsules—The Precision Modulator of Mucosal Immunity
Budesonide as the first mucosal immunomodulator approved for IgA nephropathy, employs innovative enteric-targeted delivery technology to achieve precise release in the distal ileum.
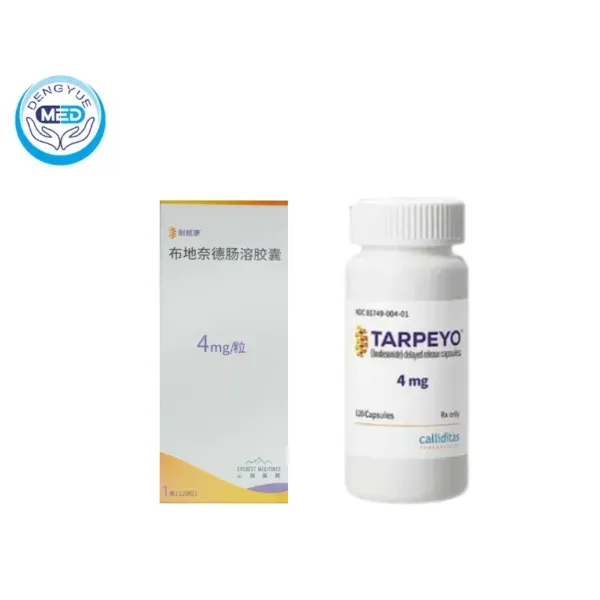
✨ Its unique value lies in directly acting on gut-associated lymphoid tissue (GALT), particularly Peyer’s patches, inhibiting the differentiation and activation of B cells producing galactose-deficient IgA1 (Gd-IgA1).
This source intervention strategy can reduce approximately 40% of pathogenic IgA production, blocking immune complex formation from the initial stage of the disease.
Clinical Evidence:
- The pivotal Phase III NefIgArd study showed that after 9 months of treatment, proteinuria decreased by an average of 34%, and the annual eGFR decline slowed from -7.3 to -2.7 mL/min/1.73 m².
- Long-term follow-up confirmed sustained efficacy, with disease progression remaining 50% slower than the control group after discontinuation.
- Subgroup analysis in Asian populations indicated particularly significant benefits for Chinese patients.
After inclusion in the national reimbursement drug list in 2024, treatment accessibility greatly improved.
Its gut-selective action reduces systemic side effects by 80% compared to traditional steroids, though monitoring of glucose metabolism and bone density indicators is necessary.
2. Sparsentan—The Dual-Pathway Synergistic Blocker
Sparsentan achieves dual receptor antagonism through a single molecule, with its structure containing high-affinity binding domains for both the angiotensin II receptor (AT1) and endothelin A receptor (ETA).

FILSPARI (Sparsentan) – IgAN | HongKong DengYue Medicine
- Generic Name/Brand Name: Sparsentan / FILSPARI®
- Indications: IgAN
- Dosage Form: Tablet
- Specification: 400 mg × 30 tablets/bottle
This dual blockade not only more comprehensively controls intraglomerular hypertension but also effectively inhibits endothelin-1-driven mesangial cell proliferation and inflammatory cytokine release.
Notably, its inhibition of the ET-1/ETA pathway can improve podocyte cytoskeletal remodeling and reduce protein leakage.
Efficacy Profile:
- The PROTECT study showed that its proteinuria-reducing effect increased in a time-dependent manner, with a 61% reduction in UPCR after 72 weeks of treatment.
- Demonstrated synergistic advantages in patients with hypertension and proteinuria.
- Renal pathology studies indicated a reduction in the proportion of glomerulosclerosis.
Adherence to the FDA black box warning is required, with monthly liver function monitoring for the first 12 months and every three months thereafter.
3. Iptacopan — The Intelligent Modulator of the Complement Cascade
Iptacopan as the first oral inhibitor of the complement alternative pathway, this drug selectively targets complement factor B, precisely regulating C3 convertase activity through allosteric inhibition.

Its uniqueness lies in inhibiting only pathological overactivation of the complement system while preserving baseline immune surveillance functions.
Recent studies reveal that it also reduces the deposition of membrane attack complexes (MAC) in glomeruli, protecting podocyte structural integrity.
Clinical Progress:
- The APPLAUSE-IgAN trial demonstrated that its proteinuria-reducing effect correlated positively with baseline complement activity.
- Subgroup analysis indicated particular efficacy in patients with C3 glomerulopathy.
- Long-term extension studies showed good tolerability with no increased risk of severe infections.
Once-daily oral dosing significantly enhances treatment convenience, with few drug interactions, making it suitable for combination therapy.
4. Telitacicept — The Systemic Regulator of B-Cell Homeostasis
This recombinant fusion protein innovatively combines the extracellular domain of the transmembrane activator (TACI) with the IgG1 Fc segment, simultaneously binding with high affinity to both BAFF and APRIL.
Taiai (Telitacicept) – SLE | HongKong DengYue Medicine
- Generic Name/Brand Name: Telitacicept/Taiai
- Indications: Autoantibody-positive systemic lupus erythematosus (SLE)
- Dosage Form: Lyophilized powder for subcutaneous injection
- Specification: 80 mg x 1 vial
Its dual inhibitory effect not only reduces mature B-cell numbers but, more critically, modulates B-cell class switching, shifting IgA production toward non-pathogenic subtypes.
Recent studies found that it also downregulates plasma cell HSP90β expression, reducing aberrantly glycosylated IgA secretion.
Efficacy Performance:
- Chinese Phase II studies indicated better outcomes in patients with high Gd-IgA1 titers.
- Immunomonitoring showed restored regulatory B-cell function after treatment.
- Significantly reduced disease exacerbations triggered by respiratory infections.
The subcutaneous injection regimen every two weeks balances efficacy and convenience, making it particularly suitable for rapidly progressing patients.
It is currently included in China’s list of clinically urgently needed overseas new drugs.
💊 These drugs each target different aspects of the disease mechanism—from mucosal immune modulation to complement inhibition, and from dual receptor blockade to B-cell regulation—together forming a comprehensive system for the precise treatment of IgA nephropathy.
Clinical selection requires comprehensive consideration of the patient’s pathological type, biomarker characteristics, and disease stage to achieve truly individualized therapy.
Future Prospects: Broader Therapeutic Horizons
Beyond the five drugs already in advanced clinical stages mentioned above, the research and development pipeline for IgA nephropathy is unprecedentedly rich:
- APRIL-Targeting Monoclonal Antibodies: Such as Sibeprenlimab, with Phase II data showing a reduction in urinary protein of up to 62%.
- Other Complement Inhibitors: Including anti-MASP-2 antibodies, C5a receptor antagonists, and more.
- CD38 Monoclonal Antibodies: Function by clearing plasma cells that produce IgA.
- Gene Therapies: Exploring the use of gene-editing technologies to correct disease-causing genetic mutations.
- Novel Immunomodulators: Such as JAK inhibitors, BTK inhibitors, and others.
These research efforts collectively point toward a future of precision medicine. In the future, doctors may select the most suitable targeted treatment plan for patients by testing biomarkers such as Gd-IgA1 levels and complement activity.
Action Guide: How Patients Should Navigate This New Era
- Doctor-Patient Communication Is Key: All treatment choices must be made under the guidance of a professional nephrologist.
While new drugs offer promise, doctors need to make comprehensive judgments based on your specific condition, pathological type, and financial situation.
- Foundational Treatment Remains Essential: Even with innovative medications, strict lifestyle management
Such as a low-salt diet, high-quality low-protein intake, and blood pressure control—along with traditional RAS inhibitor therapy, remains an indispensable cornerstone.
- Maintain Realistic Expectations: The emergence of new drugs provides more options, but they are not a cure-all.
Understand the indications, potential side effects, and treatment costs of these drugs, and work with your doctor to develop a long-term, personalized management plan.
- Seek Reliable Information: Obtain information through official channels
Such as the website of the Chinese Society of Nephrology or reputable hospital platforms, to avoid being misled by inaccurate advertisements.
Conclusion
From budesonide targeting intestinal immunity to sparsentan with dual receptor blockade, and further to iptacopan for precise complement regulation, the treatment of IgA nephropathy has entered a new era of targeted therapy.
These innovative drugs represent not merely new options but a fundamental shift in therapeutic philosophy—from passive delay to proactive intervention.
✨ Although some medications are not yet widely accessible, rapidly advancing medical technologies and increasingly robust policy support (such as insurance coverage and priority review) are accelerating the delivery of these breakthrough iga nephropathy new treatment options to more patients.
In this evolving landscape, Dengyue exemplifies how specialized pharmaceutical distribution networks are essential for translating clinical innovations into real-world therapeutic outcomes, ensuring that geographical and logistical barriers do not hinder patient care.
We invite you to share your experiences and questions about these new treatments in the comments section below.
We cordially invite you to share your experiences and insights regarding these novel treatment options in the comments section of our post titled “IgA Nephropathy: That Gut-Wrenching Moment When Hope Comes with a Hefty Price Tag.“
Your insights and discussions about real-world medication use can provide valuable perspectives for fellow patients navigating similar journeys.
FAQ about IgA Nephropathy New Treatment
What is the new treatment for IgA nephropathy 2025?
More generally, the 2025 guideline recommends that all patients with IgAN be treated with an optimized maximally tolerated dose of either an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin II receptor blocker (ARB).
Will there ever be a cure for IgA nephropathy?
There’s no cure for IgA nephropathy.
There’s also no sure way to predict how much the disease will affect your health over time.
What is the new FDA approved drug for IgA nephropathy?
The U.S. Food and Drug Administration (FDA) granted accelerated approval to Voyxact (sibeprenlimab-szsi) injection to reduce proteinuria (protein in the urine) in adults with primary immunoglobulin A nephropathy (IgAN) at risk for disease progression.
What not to eat with IgA nephropathy?
Limit salt (sodium)