How Ramucirumab Works: Precision Targeting of Tumour Angiogenesis
Tumour growth and metastasis are highly dependent on the formation of new blood vessels, a process termed tumour angiogenesis. As conventional treatment strategies face challenges such as tumour resistance and recurrence, interventions targeting angiogenesis have become a key therapeutic direction.
🎯 Vascular endothelial growth factor receptor 2 (VEGFR-2) represents a core molecular target driving tumour angiogenesis.
Upon binding its ligand VEGF, its activated downstream signalling pathways directly promote endothelial cell proliferation, migration, and neovascularisation, supplying tumours with essential nutrients and oxygen while establishing pathways for metastasis.
Consequently, precise inhibition of VEGFR-2 constitutes an effective strategy to impede tumour progression.
💊 Ramucirumab (trade name Cyramza®) is a fully humanised IgG1 monoclonal antibody. Understanding how ramucirumab works——by specifically inhibiting VEGFR-2, it achieves precise blockade of tumour angiogenesis.
Based on robust evidence from multiple global Phase III clinical trials, this drug offers a significant therapeutic option for the treatment of various advanced cancers, including gastric cancer, non-small cell lung cancer, colorectal cancer, and hepatocellular carcinoma.
Next, Hong Kong DengYue Medicine will dissect the core mechanism of action of Cyramza. Through our reliable global pharmaceutical access services, we facilitate the broader application of such breakthrough therapies in clinical practice.
Angiogenesis is the Prerequisite For Tumour Growth and Metastasis
Malignant tumours comprise large numbers of rapidly dividing and proliferating cancer cells that continuously accumulate mutations. However, tumours require a specific blood supply to obtain oxygen and other essential nutrients in order to grow to a certain size.
This process, termed tumour angiogenesis, constitutes the biological foundation enabling tumours to outgrow their microscopic dimensions, sustain continuous growth, and undergo distant metastasis.
Tumour cells secrete signalling proteins such as vascular endothelial growth factor (VEGF) in substantial quantities. These proteins act as ‘distress signals,’ binding to specific receptors on the surface of vascular endothelial cells (primarily VEGFR-2).
Upon binding, they initiate complex intracellular signalling pathways that ultimately stimulate endothelial cell proliferation, migration, and the formation of new, often structurally disorganised tumour vessels.
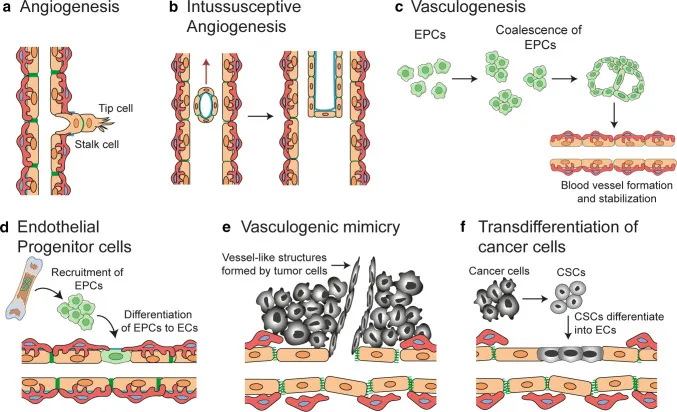
✨ These newly formed vessels not only supply the tumour with oxygen and nutrients but also serve as conduits for cancer cells to enter the circulatory system and disseminate throughout the body.
Core Interpretation——How Ramucirumab Inhibits Angiogenesis by Blocking VEGFR-2?
Interfering with the aforementioned angiogenesis process is how ramucirumab works. It is a humanised immunoglobulin G1 monoclonal antibody specifically targeting VEGFR-2.
Unlike certain small-molecule inhibitors, Cyramza ramucirumab binds directly to a specific epitope on the extracellular domain of VEGFR-2, exhibiting a highly specific mode of action.
This binding prevents the attachment of its ligands (VEGF-A, VEGF-C, and VEGF-D), functioning much like inserting a high-affinity ‘molecular lock’ into a keyhole.
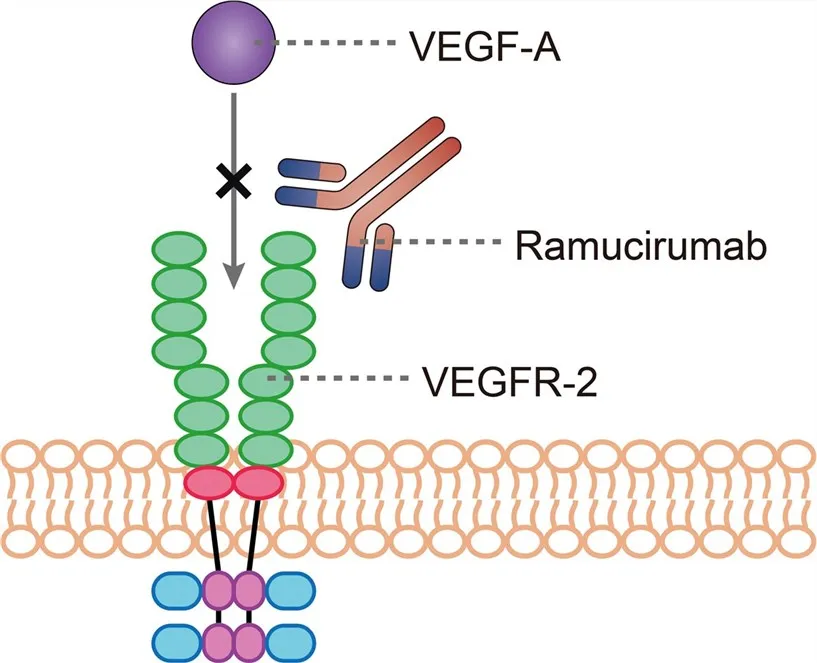
Consequently, ligand-induced receptor phosphorylation is blocked, effectively interrupting the downstream cascade of signalling that drives endothelial cell proliferation, increases vascular permeability, and promotes cell migration.
👉 Cyramza ramucirumab reduces tumour angiogenesis and exacerbates tumour hypoxia by inhibiting VEGFR-2, thereby enhancing the efficacy of chemotherapy. It has become a pivotal therapeutic agent for combating advanced and drug-resistant cancers.
Clinically Proven Therapeutic Efficacy
- REGARD Trial
Study Population: Patients with advanced or metastatic gastric cancer or adenocarcinoma of the gastroesophageal junction.
Treatment Regimen: Cyramza monotherapy vs placebo.
Key Findings: Cyramza monotherapy significantly prolonged overall survival (OS) compared with placebo.
- RAINBOW Trial
Study Population: Patients with advanced or metastatic gastric cancer or adenocarcinoma of the gastroesophageal junction.
Treatment Regimen: Cyramza plus paclitaxel versus placebo plus paclitaxel.
Key Findings: The Cyramza combination therapy group demonstrated statistically significant improvements in both overall survival (OS) and progression-free survival (PFS) .
- REACH-2 Trial
Study Population: Patients with hepatocellular carcinoma who had received prior sorafenib therapy and exhibited alpha-fetoprotein concentrations ≥400 ng/mL.
Treatment Regimen: Cyramza versus placebo.
Key Findings: The Cyramza treatment group demonstrated significantly superior overall survival (OS) and progression-free survival (PFS) compared to the placebo group.
Ramucirumab vs Bevacizumab:Comparison of Two Anti-Angiogenic Drugs
In the treatment of EGFR-mutant non-small cell lung cancer (NSCLC), the core distinction between the two primary anti-angiogenic agents, ramucirumab and bevacizumab, lies in their differing points of intervention within the tumour angiogenesis signalling pathway.
In layman’s terms, VEGF-A is the ‘command signal’ driving angiogenesis, while VEGFR-2 is the ‘key switch’ receiving the command.
👉 Bevacizumab acts upon the ‘command signal’ by binding to and neutralising VEGF-A, thereby preventing signal transmission.
👉 How ramucirumab works is binding with high affinity to the extracellular domain of VEGFR-2, directly targeting the “key switch” to block the binding of multiple VEGF ligands to their receptors.
| Comparative drug | Ramucirumab | Bevacizumab |
| Target site | VEGFR-2 | VEGF-A |
| Core mechanism of action | Directly binds to the extracellular domain of VEGFR-2, physically preventing multiple ligands including VEGF-A, VEGF-C, and VEGF-D from binding to the receptor. | Binds to and neutralises VEGF-A protein in the circulation, preventing its binding to receptors. |
| Signal Interception Levels | Block the reception of all relevant signals (VEGF-A/C/D) at the receptor level | Blocking the transmission of specific signals (VEGF-A) at the extracellular level |
| Primary approved indications | Combination chemotherapy or targeted therapy for advanced gastric cancer/gastroesophageal junction adenocarcinoma, non-small cell lung cancer, colorectal cancer, and hepatocellular carcinoma (AFP ≥ 400 ng/mL) | Combination chemotherapy or targeted therapy for non-squamous non-small cell lung cancer, colorectal cancer, glioblastoma, ovarian cancer, and other conditions. |
From a clinical perspective, when used in combination with EGFR-TKI as first-line therapy for advanced EGFR-mutant NSCLC, both drugs demonstrated comparable efficacy in prolonging progression-free survival (PFS) and overall survival (OS), with broadly similar safety profiles.
This indicates that although they intervene at distinct points within the signalling pathway, they ultimately achieve similar outcomes in inhibiting tumour angiogenesis and synergistically enhancing the efficacy of targeted therapy.
Although current clinical data indicate comparable efficacy, exploring subtle differences between them in distinct patient subgroups and under varying resistance contexts remains a crucial avenue for future precision medicine research.
Clinical Application of Ramucirumab — Primarily Covering Four Types of Advanced Solid Tumours
1. Advanced Gastric Cancer and Gastro-oesophageal Junction Cancer
For patients with advanced gastric cancer or adenocarcinoma of the gastro-oesophageal junction who have progressed following prior fluorouracil- or platinum-based chemotherapy, it may be administered as monotherapy or in combination with paclitaxel.
The aforementioned RAINBOW trial demonstrated that combining Cyramza injection with paclitaxel significantly improved overall survival compared to paclitaxel monotherapy.
2. Hepatocellular Carcinoma (HCC)
For patients with hepatocellular carcinoma (HCC) who have previously received sorafenib treatment and have alpha-fetoprotein (AFP) ≥400 ng/mL, it may be used as monotherapy.
The aforementioned REACH-2 trial demonstrated improved survival rates in patients meeting these criteria.
3. Non-Small Cell Lung Cancer (NSCLC)
Indicated for use in combination with docetaxel for the treatment of metastatic non-small cell lung cancer patients whose disease has progressed following platinum-based chemotherapy.
Clinical trials indicate prolonged survival benefit, particularly in previously treated non-small cell lung cancer patients.
4. Colorectal Cancer (mCRC)
For use in combination with FOLFIRI (an irinotecan-based chemotherapy) in patients with metastatic colorectal cancer who have developed resistance to bevacizumab therapy.
Studies indicate Cyramza 500mg may benefit patients who have developed resistance to other VEGF-targeted therapies.
The clinical application prospects for Cyramza continue to expand. Presently, its research scope extends beyond approved indications to encompass bladder cancer, pancreatic cancer, and other tumours expressing VEGFR-2.
Precautions Before Using Medication
1️⃣ Consult Official Medicinal Product Information
We strongly advise you to visit the official website of a medicinal products regulatory authority to access the latest official product information for Cyramza 500.
The most authoritative information on how Ramucirumab works, its indications, dosage and administration methods, safety data, and other key details.
2️⃣ Seek Professional Medical Institutions
Treatment decisions for complex tumours should be made at medical centres with extensive experience. You may search for and formally contact nationally accredited cancer centres or oncology departments within major tertiary hospitals. These institutions typically possess multidisciplinary teams capable of developing comprehensive treatment plans for patients.
3️⃣ Prepare a Personalised List of Questions
❓ Prior to discussions with your attending physician, we advise preparing a list of questions tailored to your specific circumstances, such as:
- ‘What is the specific treatment regimen (monotherapy or combination therapy)? What are the anticipated treatment objectives?’
- ‘What potential adverse reactions should we jointly monitor? How should we respond if they occur?’
- ‘How is the approximate treatment cycle structured, and when will efficacy be assessed?’
4️⃣ Prioritise Ongoing Patient Support
Research reports on Cyramza may be accessed via the official website of the pharmaceutical manufacturer Eli Lilly.
Moreover, authoritative medical platforms such as DengYue Medicine also provide updates on cancer treatment products and therapeutic guidelines presented in accessible language.
The Global Clinical Value and Public Health Significance of Ramucirumab
✅ Establishing New Treatment Standards for Refractory Tumours
As a monoclonal antibody specifically targeting VEGFR-2, Cyramza’s success has validated this target’s core therapeutic value across multiple solid tumours.
Its positive Phase III clinical outcomes in poor-prognosis settings such as advanced gastric cancer and hepatocellular carcinoma (in patients with alpha-fetoprotein ≥400 ng/mL) have prompted updates to treatment guidelines by major regulatory authorities including the US, EU, and China.
This provides clinicians globally with high-level evidence-based medical support, standardising late-line treatment for such patient populations.
✅ Delivering Predictable Health Economic Value
In comparison with novel tumor immunotherapies or cell therapies, How Ramucirumab Works, along with its relatively well-defined biomarkers for predicting therapeutic efficacy, allows for more precise patient selection and prediction of treatment outcomes.
This characteristic facilitates more accurate pharmacoeconomic evaluations by health technology assessment bodies worldwide, thereby influencing reimbursement policy formulation and enhancing drug accessibility and sustainability across diverse healthcare systems.
✅ Driving Innovation in Combination Therapy Development Paradigms
Research into combinations of Cyramza ramucirumab with chemotherapy, targeted therapies, and even immunotherapies has established a paradigm for the concept of ‘anti-angiogenic agents as therapeutic cornerstones.’
Its approval in combination with erlotinib for EGFR-mutated non-small cell lung cancer further solidified the synergistic model of ‘targeted driver gene therapy + anti-angiogenesis.’
These successful combination experiences are now inspiring and guiding the development of similar combination regimens for other cancer types globally, amplifying their scientific contributions.
DengYue Medicine will continue to monitor cutting-edge developments in this field, committed to presenting and interpreting the latest research findings and treatment strategies for healthcare professionals and patients.
FAQ about How Ramucirumab Works
What is the mechanism of action of ramucirumab?
Cyramza ramucirumab is a fully human monoclonal antibody (IgG1) that specifically targets VEGFR-2, thereby preventing VEGF ligands from activating VEGFR-2.
Is ramucirumab a VEGF inhibitor?
Cyramza ramucirumab is a direct inhibitor of VEGFR-2, binding with high specificity to the extracellular VEGF-binding domain and preventing VEGF ligands from binding to the VEGF-R2 receptor.
What type of drug is ramucirumab?
Cyramza ramucirumab is a targeted therapy drug classified as an angiogenesis inhibitor. It functions by blocking a protein known as VEGFR-2, which is produced in large quantities by certain cancer cells.
What is the mechanism of action of VEGF inhibitors?
Inhibition of VEGF signalling can influence tumour growth and progression through multiple mechanisms, such as suppressing angiogenesis, inducing neovascularisation regression, and altering tumour vascular function.