From Generics to Bio-Engineering: How China is Redesigning the Future of Medicine
For decades, “Made in China” in pharmaceuticals conjured images of generic drugs and manufacturing scale.
Today, that narrative is obsolete. A quiet revolution is reshaping the global biotech landscape, driven by China’s emergence as a powerhouse in drug bio-engineering the deliberate, precision design of next-generation therapeutics.
This isn’t just about making existing drugs cheaper; it’s about architecting better ones from the molecule up.
The proof? According to data compiled by Hong Kong DengYue Medicine, Landmark license-out deals are where global giants like Pfizer, Merck, and Bristol-Myers Squibb are paying billions—$1.25B, $1.7B, and $8B upfront—not for sales rights in China, but for Chinese-designed drugs to sell worldwide.
This is the definitive journey “From Generics to Bio-Engineering.” It’s a transition from volume to value, from replication to origination.
🤔 But what fuels this leap?
The stage is set for a profound transformation. This evolution from generics to bio-engineering demands we first dissect the intellectual revolution at its core: the shift from discovering drugs to designing them.
🙂 Let’s examine the blueprint of this bio-engineered future.
The Paradigm Shift: From Serendipity to Precision Design
Classic drug discovery often relied on serendipity and mass screening—finding a molecule that fits a target.
This “needle-in-a-haystack” approach, while successful, was slow and inefficient for complex diseases like cancer or autoimmune disorders.
The resulting drugs were frequently single-target agents, like a key for one lock.
The Rise of Precision Bio-Engineering
Bio-engineering represents a fundamental paradigm shift. Instead of just finding molecules, scientists now design them.
Using advanced computational tools, structural biology, and synthetic techniques, they build multi-functional therapeutic proteins and conjugates with specific properties.
Think of it as the difference between a blacksmith forging a tool and an engineer designing a multi-tool at the atomic level.
This approach is crucial for developing next-generation therapeutics that tackle disease through multiple pathways simultaneously.
Why China Excels in This New Paradigm
China’s ascent in bio-engineering is no accident.
It’s the product of a perfect storm: a massive reverse “brain drain” of Western-trained scientists, a world-class CXO infrastructure (like WuXi and Pharmaron) that acts as a biotech “foundry,” and a dynamic domestic market that allows for rapid clinical validation.
The government’s strategic focus on biotech innovation has further fueled R&D investment, creating an ecosystem optimized for the iterative, platform-based nature of modern drug design. This is the operational foundation of the leap from generics to bio-engineering.
This conceptual shift from discovery to design is not merely theoretical.
It has materialized into a portfolio of tangible, sophisticated drugs that are already altering treatment paradigms and global partnership dynamics.
The proof lies in three pivotal battlefields of modern therapeutics.🔻
Proof on the Battlefield: China’s Bio-Engineering in Action
The theoretical shift to bio-engineering is proven by tangible, groundbreaking drugs. China’s prowess is most evident in three transformative classes of next-generation therapeutics.
Bispecific Antibodies: Engineering “Two-in-One” Smart Weapons
Bispecific antibodies are a prime achievement of bio-engineering. By designing a single protein that binds two different targets, they coordinate a more potent and specific attack on disease.
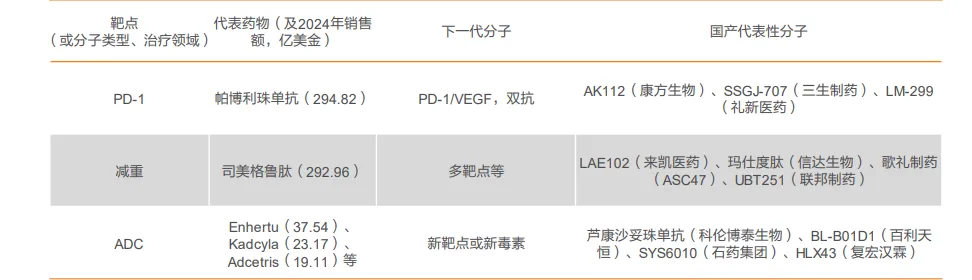
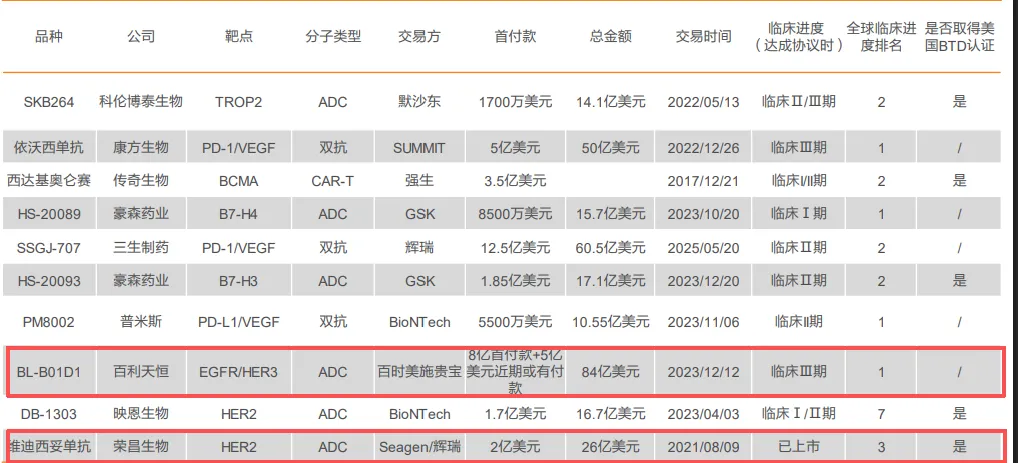
- The Flagship Achievement: Akeso’s Ivonescimab is the world’s first approved tumor immunotherapy bispecific antibody targeting PD-1 and VEGF.
👉 This bio-engineered molecule simultaneously unlocks the immune system and starves the tumor of blood supply. Its $500 million upfront license-out deal signaled China’s arrival as a leader in complex protein design.
- The Commercial Validation: 3SBio’s SSGJ-707, a similar PD-1/VEGF bispecific.
👉 It recently secured a staggering $1.25 billion upfront from Pfizer—one of the largest deals of its kind—while still in mid-stage trials. This isn’t just an investment in a drug; it’s an investment in China’s bispecific antibody platform and its bio-engineering credibility.
Antibody-Drug Conjugates (ADCs): Engineering “Precision-Guided Missiles”
ADC technology is the pinnacle of controlled drug design, requiring perfect harmony between an antibody (the guidance system), a linker (the safety), and a cytotoxic payload (the warhead).
- The Masterpiece of Complexity: Biotherapeutics’ BL-B01D1 is a globally first-in-class (FIC), dual-targeting ADC against EGFR and HER3.
👉 This molecule represents a staggering feat of bio-engineering, combining two targeting motifs with a novel toxin.
💰 Its total potential deal value of $8.4 billion with Bristol-Myers Squibb underscores the immense value placed on such sophisticated drug design platforms.
- Platform Validation: Companies like RemeGen (with its HER2 ADC Disitamab Vedotin, out-licensed to Seagen)
👉 It has proven the robustness of China’s ADC platforms, moving beyond following established targets to innovating in novel linkers and payloads.
Beyond Antibodies: Engineering Complex Biological Systems
The frontier of bio-engineering extends into modulating entire biological systems, as seen in obesity and advanced immunotherapy.
- Multi-Target Agonists for Obesity: Innovent’s Mazdutide is a bio-engineered dual agonist targeting GLP-1 and GIP receptors for weight loss.
👉 Its promising clinical data shows China can compete head-to-head with global leaders in one of the hottest therapeutic areas, moving beyond single-target GLP-1 drugs.
Taming Cytokines: A Breakthrough in Immunotherapy: Cytokines like IL-2 are powerful immune stimulants but notoriously toxic.
✨ Innovent’s PD-1/IL-2α fusion protein is a groundbreaking piece of bio-engineering that “masks” the cytokine’s harmful side effects while preserving its anti-tumor activity.
👉 This solves a decades-old problem in immunotherapy and exemplifies first-in-class innovation from China.
The triumphs in bispecifics, ADCs, and complex biologics are not isolated miracles. They are the outputs of a matured and uniquely supportive national ecosystem.
🎯 To understand the sustainability of China’s bio-engineering rise, we must look at the engine room that powers it.
The Engine Room: Ecosystem Fueling Bio-Engineering Dominance
This bio-engineering output is not a fluke but the product of a matured ecosystem.
- The Talent: A generation of scientists with experience at top global pharma and biotech firms now lead R&D in China, blending global insight with local execution speed.
- The Infrastructure: China’s CXO sector provides unparalleled efficiency in turning molecular designs into clinical-grade material, dramatically lowering the barrier for innovation.
- The Capital & Policy: Friendly listing rules (HKEX 18A, STAR Market) and government full-chain support policies provide the fuel and regulatory runway for high-risk, high-reward bio-engineering projects.
With this powerful ecosystem consistently translating bold designs into validated drugs and major deals, the implications for the global biotech order are profound.
😉 The journey we began by tracing is now reaching its logical conclusion.
Conclusion: Redefining the Global Biotech Competition
The story of Chinese biopharma has been rewritten. The competitive edge is no longer just cost or speed, but intellectual property in drug design. The future belongs to those who can best bio-engineer solutions to biological complexity.
The massive license-out deals are more than paychecks; they are royalties on China’s bio-engineering intellectual property.
They mark a shift from a generics mindset to an innovation exporter model. This transformation extends beyond the lab to the entire value chain.
✨ Companies like DengYue Medicine, traditionally known as a pharmaceutical distributor, are now evolving into critical partners for bio-engineered therapies.

✅ By leveraging their deep commercial networks and market expertise, they help bridge the gap between groundbreaking drug design and patient access, ensuring that these complex, next-generation therapeutics reach the clinics and patients who need them most efficiently.
This synergy between innovative R&D and smart commercialization completes the ecosystem, turning scientific breakthroughs into tangible health impact.
🎯 When you think of the future of precision oncology, targeted therapies, and next-generation therapeutics, you should now look to China—not just as a market, but as a primary source of bio-engineering innovation.
The molecules being designed in labs in Shanghai, Chengdu, and Suzhou today are not “Chinese drugs”; they are the globally-first-in-class medicines of tomorrow, designed in China.
🌐 The game has changed, and China is helping write the new rules—from discovery and design all the way to distribution and delivery.
FAQ about From Generics to Bio-Engineering
What is the difference between biologics and generics?
Biosimilars are imitations of reference products called “biologics,” which are made up of living cells.
Generics are copies of reference medicines that are composed of chemicals.
A drug cannot have both a biosimilar and a generic. There can only be one or the other.
Why don’t biologics have generics?
Biologics are made from living sources and have complex structures. Biosimilar and generic drugs are made and regulated differently.
But they can both help you save money on your prescription.
Are there any generic biologics?
They are called biosimilars. It’s common to think of biosimilars as generic biologics, but that isn’t exactly right.
Which is better, generic or non-generic?
A generic drug must be “bioequivalent” to the brand-name product, meaning they have to be chemically similar.
A recent study that compared generics to brand-name drugs found on average only a 3.5% difference in absorption into the body