
Biktarvy: How Does a Three-Drug-in-One Regimen Define the Gold Standard in HIV Treatment?
In the field of HIV antiviral therapy, “simplification” and “potency” have long been the two enduring goals that define the gold standard in HIV treatment.
A prime example that perfectly integrates both is Biktarvy®—a single-tablet regimen combining three agents in one pill.
✨ It is not only recommended as a first-line preferred option in major international guidelines but is also regarded by many clinicians and patients as a “gold standard” for long-term HIV management.
What gives this “gold standard” its true value? The answer lies in the synergistic mechanism of its three components, where “1 + 1 + 1 > 3.”
Following DengYue Medicine, this article explores in depth how these three forces work in precise coordination to achieve potent and durable suppression of HIV replication.
Getting to the Core—What Is Biktarvy?
Biktarvy is a fixed-dose, single-tablet combination regimen indicated for the treatment of HIV-1 infection in adults and adolescents weighing ≥25 kg.
It is primarily used in two clinical scenarios:
- Treatment-naïve patients: As a preferred first-line option for initiating antiretroviral therapy.
- Treatment-experienced patients switching therapy: For patients who have received prior treatment and achieved sustained virologic suppression (HIV-1 RNA <50 copies/mL) for at least 3 months, with no history of treatment failure and no known resistance to any component of Biktarvy, the regimen may be used to simplify or optimize therapy.

Key Features
- A Potent Triple-Drug Combination
Biktarvy integrates the next-generation integrase strand transfer inhibitor bictegravir (50 mg) with an optimized NRTI backbone of emtricitabine (200 mg) and tenofovir alafenamide (25 mg).
✨ Each component is considered a best-in-class agent within its category, together forming an elite regimen in modern HIV therapy.
- Robust Efficacy Data
Pivotal Phase III clinical trials demonstrated virologic suppression rates at Week 48 of 92.4% in treatment-naïve patients and 93.6% in switch patients.
In long-term studies extending to 144 weeks, high levels of viral suppression were maintained, confirming the durable and potent antiviral activity of Biktarvy.
- Favorable Safety and Tolerability Profile
Compared with traditional tenofovir disoproxil fumarate (TDF)–based regimens, Biktarvy showed significant safety advantages at 144 weeks:
- Lumbar spine bone mineral density increased by 1.9% (vs. a 0.4% decrease in the TDF group)
- Total hip bone mineral density increased by 1.4% (vs. a 0.8% decrease in the TDF group)
- A significantly smaller decline in estimated glomerular filtration rate (eGFR)
Across studies, discontinuation due to adverse events occurred in fewer than 2% of patients, indicating excellent long-term tolerability.
- An Ultra-Simple Dosing Regimen
Biktarvy is taken as one tablet once daily, with or without food. 🥗
Studies show that, compared with multi-tablet regimens, single-tablet regimens improve adherence by approximately 20%.
✨ A systematic review further demonstrated that simplification to a single-tablet regimen significantly reduces treatment fatigue and can lower missed-dose rates by up to 30%—a critical advantage in lifelong HIV management, where adherence is central to long-term success.
Taken together, Biktarvy establishes a near-ideal HIV treatment framework built on four key pillars: carefully selected components, potent and durable efficacy, optimized long-term safety, and extreme dosing simplicity.
In the next section, we will delve deeper into the mechanisms of action behind this “three-in-one” regimen to understand how its components collaborate at critical stages of viral replication, achieving a true 1 + 1 + 1 > 3 effect. ❓
Mechanistic Depth—How Do the Three Components Work in Synergy?
The core strategy of modern HIV therapy is multi-target combination treatment, designed to maximally suppress viral replication while preventing the emergence of drug resistance.
The three active components of Biktarvy®—bictegravir (BIC), emtricitabine (FTC), and tenofovir alafenamide (TAF)—form a complementary and synergistic inhibitory network at two critical stages of the HIV replication cycle.

1️⃣ The First Line of Defense: Dual Inhibition of Reverse Transcription
Both emtricitabine and tenofovir alafenamide are nucleos(t)ide reverse transcriptase inhibitors (NRTIs) that act through competitive inhibition of HIV-1 reverse transcriptase.
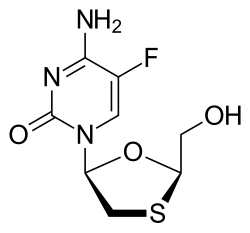
🧬 When the enzyme mistakenly incorporates these nucleotide analogs into the nascent viral DNA strand, premature chain termination occurs, effectively halting DNA synthesis.
- Clinically proven synergy: The group of FTC and tenofovir alafenamide has been validated through more than 15 years of clinical use, demonstrating robust efficacy across millions of patients worldwide and establishing itself as a cornerstone NRTI combination.
- Pharmacokinetic breakthrough of TAF: As a novel prodrug of tenofovir, TAF exhibits markedly improved plasma stability and is more efficiently taken up by target cells, particularly CD4⁺ T lymphocytes.
Pharmacokinetic studies show that intracellular concentrations of tenofovir diphosphate achieved with TAF are more than fourfold higher than those with traditional tenofovir disoproxil fumarate (TDF), while plasma exposure is reduced to approximately one-tenth.
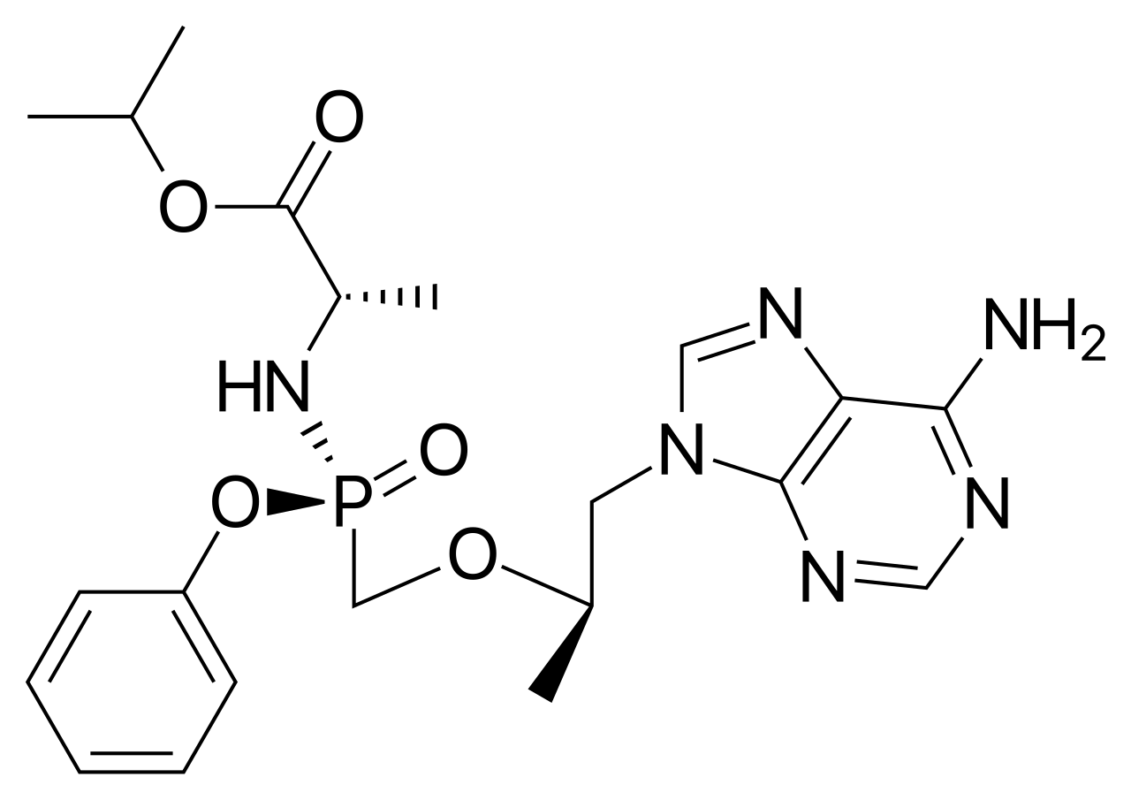
✨ This differentiated tissue distribution translates directly into clinical benefits: in pivotal Phase III trials, the incidence of renal dysfunction (≥25% decline in creatinine clearance) was 32% lower in the TAF group than in the TDF group, accompanied by significantly improved bone safety outcomes.
2️⃣ The Second Line of Defense: Innovative Blockade of Viral Integration
Bictegravir is a second-generation integrase strand transfer inhibitor (INSTI).
👉 It inhibits HIV-1 integrase by forming coordination bonds with metal ions at the enzyme’s active site, stabilizing the integrase–viral DNA complex and preventing integration of viral DNA into the host genome.
- Unique allosteric inhibition: Beyond competitive inhibition, bictegravir induces the formation of a non-productive, catalytically inactive conformation of the integrase–DNA complex.
✅ This allosteric effect further enhances its antiviral potency.
- Exceptional potency and high resistance barrier: In vitro studies demonstrate an EC₅₀ of 0.51 nM, reflecting extremely strong antiviral activity.
✅ Across clinical trials with follow-up extending beyond 144 weeks, no treatment-emergent integrase resistance mutations associated with bictegravir were observed, a finding attributed to its distinct chemical structure and efficient target engagement.
- Rapid virologic response: INSTI-based regimens typically achieve a >2 log₁₀ reduction in viral load within 4–8 weeks of treatment initiation.
✅ Bictegravir-based therapy consistently demonstrates this rapid onset of action.
3️⃣ Scientific Basis and Clinical Value of Synergy
- Pharmacodynamic Principles of Synergy
- Spatially sequential blockade: FTC/TAF inhibits reverse transcription in the cytoplasm, while bictegravir blocks viral DNA integration in the nucleus, creating a three-dimensional, sequential barrier against HIV replication.
- Multiple viral targets: By simultaneously targeting reverse transcriptase and integrase, the regimen disrupts viral replication through distinct yet complementary mechanisms.
👉 Mathematical modeling indicates that multi-target combination therapy reduces the probability of resistance development by at least three orders of magnitude compared with monotherapy.
- Clinical Translation
- Depth and durability of viral suppression: Under this triple-mechanism synergy, the median time to achieve HIV-1 RNA <50 copies/mL is approximately 4 weeks, with very low long-term virologic failure rates.
- Prevention of resistance development: In pivotal clinical studies, fewer than 0.5% of patients developed treatment-emergent resistance mutations, primarily affecting NRTI targets and not compromising ongoing integrase inhibition.
- Regimen stability in real-world practice: Real-world data show that fewer than 2% of patients receiving Biktarvy switch treatment due to virologic failure—significantly lower than rates observed with traditional multi-tablet regimens.
Through precise intervention across the HIV replication cycle and multi-layered molecular protection, Biktarvy achieves not only theoretical synergistic enhancement but also clinically validated superiority.
✨ This integration of potent antiviral activity, a high resistance barrier, and long-term regimen stability underpins its recognized role as a modern gold standard in HIV treatment.
From Mechanism to Meaningful Benefits—Real-World Impact for Patients
Built on its unique triple-synergy mechanism, Biktarvy® not only delivers strong clinical trial results, but also brings substantial, multidimensional improvements to patients’ real-world treatment experience and long-term health management.
👉 These benefits directly influence clinical outcomes, psychological well-being, and overall quality of life.
Reaching “U=U” Faster — Restoring Confidence and Social Connection
Key studies show that patients receiving potent integrase inhibitor–based regimens, including Biktarvy, achieve HIV-1 RNA <50 copies/mL in a median time of approximately 4 weeks.
Compared with some traditional regimens, this represents a significantly faster path to an undetectable viral load.
👉 “U=U” (Undetectable = Untransmittable) is a public-health consensus supported by extensive scientific evidence. Achieving this status earlier and more reliably has profound implications for patients:
- 🥰 Psychological liberation: It markedly reduces long-standing anxiety about transmission, feelings of shame, and internalized stigma—representing a critical step in psychological recovery.
- 🤝 Empowerment in relationships: With sustained viral suppression and good adherence, patients gain a solid scientific foundation for establishing or maintaining intimate relationships, helping to rebuild social support networks.
- 🤰 Reproductive choices: It creates the essential conditions for safe conception, whether through natural conception in serodiscordant couples or with assisted reproductive technologies, enabling the birth of HIV-negative children.
A “Long-Term Anchor” for Lifelong Management
As discussed earlier, the high genetic barrier to resistance provided by Biktarvy’s triple-mechanism synergy is fundamental to long-term treatment success.
Real-world data show virologic failure rates of less than 1% after one year of treatment, offering patients greater certainty and reducing anxiety about long-term loss of efficacy.
🫀 Renal and bone toxicity associated with older therapies has long been a “silent threat” in lifelong HIV treatment, often leading to late regimen changes or complications.
The inclusion of TAF in Biktarvy significantly mitigates these long-term risks:
- Renal protection: Studies indicate that the incidence of chronic kidney disease is reduced by approximately 65% with TAF-based regimens compared with TDF.
- Bone safety: Long-term risks of osteoporosis and fractures are substantially lower.
These advantages are particularly important for patients with comorbidities such as hypertension or diabetes, which further increase renal risk.
They also allow younger patients to plan decades of treatment with greater confidence, reducing the likelihood of side-effect-driven regimen changes that could compromise efficacy.
Dual Gains in Quality of Life and Adherence — A Return to Normalcy
- The once-daily, single-tablet, food-independent regimen respects patients’ daily lives.
Studies show that compared with multi-tablet regimens, single-tablet regimens improve medication adherence (PDC ≥90%) by approximately 20–30%.
High adherence remains the most important modifiable factor for sustained viral suppression and resistance prevention.
- Simplifying and discreet dosing significantly reduces the constant reminder of illness.
Patients no longer need to manage complex dosing schedules at work, during travel, or in social settings, allowing them to focus more fully on living.
✅ Higher treatment satisfaction is directly associated with better mental health and improved long-term outcomes.
- Treatment simplification may also indirectly reduce work absenteeism and productivity loss associated with illness and complex regimens.
Stable health enables patients to remain engaged in work, education, and social roles, enhancing social participation and personal fulfillment.
In summary, the pathway from mechanism to meaningful benefit is both clear and compelling.
Together, these benefits are helping an increasing number of people living with HIV seamlessly integrate chronic disease management into a long-term pursuit of health, fulfillment, and dignity.
Conclusion
The success of Biktarvy® exemplifies the core principles of modern antiviral drug design: by optimally combining three highly effective agents with complementary mechanisms of action, it achieves the ideal balance of synergistic efficacy and toxicity mitigation.
🎯 Biktarvy is not merely a tablet—it is a carefully engineered treatment system, precisely designed to deliver durable viral suppression while supporting long-term safety and adherence.
As a trusted healthcare and global pharma partner, DengYue Medicine remains committed to providing high-quality antiviral therapies, including Biktarvy, alongside professional health management support for healthcare institutions and patients alike.
🙂 We firmly believe that the true value of innovative therapies lies not only in the medicines themselves but in ensuring that they are safe, accessible, and appropriately used in clinical practice, ultimately translating scientific advances into tangible patient benefits.

Biktarvy (Bictegravir/Emtricitabine/TAF) – HIV-1 | HongKong DengYue Medicine
- Generic Name/Brand Name: Bictegravir, Emtricitabine, Tenofovir Alafenamide/Biktarvy
- Indications: HIV-1
- Dosage Form: Film-coated tablet
- Specification: 50 mg/200 mg/25 mg × 30 tablets
FAQ about The Gold Standard in HIV Treatment
What is Biktarvy used for?
Biktarvy is an antiviral medicine used to treat adults and children from 2 years of age and weighing at least 14 kg infected with human immunodeficiency virus 1 (HIV-1).
Is Biktarvy a high-risk drug?
No, but Biktarvy can cause serious, life-threatening side effects.
Does Biktarvy make you undetectable?
The 3 powerful medicines in BIKTARVY were designed to attack the virus right away and to work together to lower the amount of HIV in the blood to undetectable levels.
What are the main side effects of Biktarvy?
The most common side effects of BIKTARVY in clinical studies were diarrhea (6%), nausea (6%), and headache (5%).



