Stivarga (Regorafenib) – Colorectal cancer | HongKong DengYue Medicine
- Generic Name/Brand Name: Regorafenib/Stivarga
- Indications: Metastatic colorectal cancer, Gastrointestinal stromal tumors (GIST), Hepatocellular carcinoma (HCC), Renal cell carcinoma (RCC)
- Dosage Form: Oral Tablets
- Specification: 40mg x 28 tablets
Regorafenib Application Scope
Regorafenib is a multi-kinase inhibitor that targets several pathways involved in tumor growth and cancer spread. It’s primarily used for the treatment of:
-
Colorectal Cancer (metastatic)
-
Gastrointestinal Stromal Tumors (GIST) (after failure of prior treatment)
-
Hepatocellular Carcinoma (HCC)
-
Renal Cell Carcinoma (RCC)
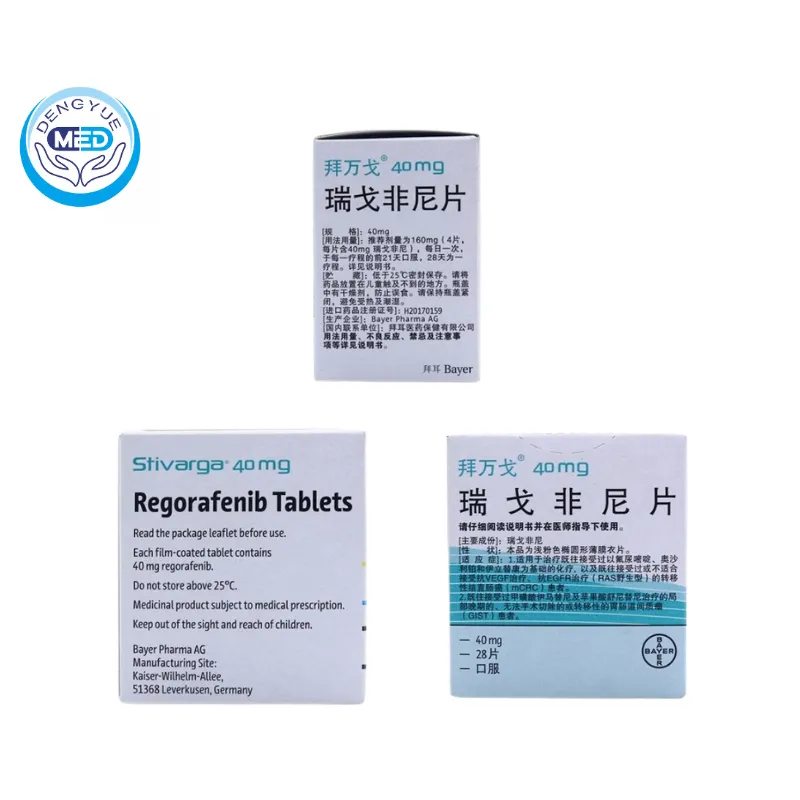
Regorafenib Characteristics
-
Ingredients: The active ingredient is Regorafenib.
-
Properties: Regorafenib acts by inhibiting several kinases involved in tumor growth, angiogenesis, and metastasis. These include VEGFR, PDGFR, KIT, and RET, among others.
-
Packaging Specification: Typically available in tablet form, with a strength of 40 mg per tablet.
-
Storage: Store at room temperature, away from moisture and heat.
-
Expiry Date: Refer to the packaging for the expiry date. Generally, it is 2-3 years from the manufacturing date.
-
Executive Standard: Based on local health regulatory standards, such as the US FDA or EMA for Europe.
-
Approval Number: The specific approval number can be found on the drug’s packaging, subject to local regulatory bodies.
-
Date of Revision: As indicated by the latest update from the manufacturer or regulatory authorities.
-
Manufacturer: Stivarga
Guidelines for the Use of Regorafenib
-
Dosage and Administration:
-
Recommended Dose: Typically, 160 mg/day, taken orally once daily for 21 days of a 28-day cycle. However, dose adjustments are common based on tolerability and side effects.
-
Administration: Taken with a low-fat meal to reduce gastrointestinal side effects. Swallow tablets whole without crushing or chewing.
-
Missed Dose: If a dose is missed, skip it and take the next dose at the usual time. Do not double the dose to make up for a missed dose.
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
Fatigue
-
Diarrhea
-
Hypertension
-
Rash
-
Hand-foot skin reactions (palmar-plantar erythrodysesthesia)
-
-
Serious Adverse Reactions:
-
Severe liver toxicity (hepatotoxicity)
-
Gastrointestinal perforation
-
Hemorrhage
-
Reversible posterior leukoencephalopathy syndrome (RPLS)
-
Myocardial infarction (heart attack)
-
Severe bleeding (including nosebleeds)
-
-
-
Contraindications:
-
Known hypersensitivity to Regorafenib or any of its components.
-
Severe liver impairment (Child-Pugh Class C).
-
Pregnancy (Category D – avoid use during pregnancy).
-
-
Precautions:
-
Monitor liver function before and during treatment.
-
Hypertension: Control prior to starting treatment and monitor regularly.
-
Hemorrhagic Events: Regular monitoring for signs of bleeding.
-
Use with caution in patients with cardiac or vascular diseases.
-
Regorafenib Interactions
-
CYP3A4 Inhibitors/Inducers: May affect Regorafenib levels. Drugs that inhibit or induce CYP3A4 can alter the effectiveness of Regorafenib.
-
Warfarin and other anticoagulants: Increased risk of bleeding.
-
Antihypertensive drugs: Potentiation of anti-hypertensive effects, increasing the risk of hypotension.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.



Reviews
There are no reviews yet.