Pegpesen (Inpegsomatropin Injection) – AGHD | HongKong DengYue Medicine
- Generic Name/Brand Name: Inpegsomatropin Injection/Pegpesen
- Indications: AGHD
- Dosage Form: Injection
- Specification: 5 mg x 1 syringe
Inpegsomatropin Injection Application Scope
Inpegsomatropin Injection is indicated for the treatment of growth hormone deficiency (GHD) in pediatric and adult patients, as well as other approved conditions where recombinant human growth hormone therapy is clinically required.
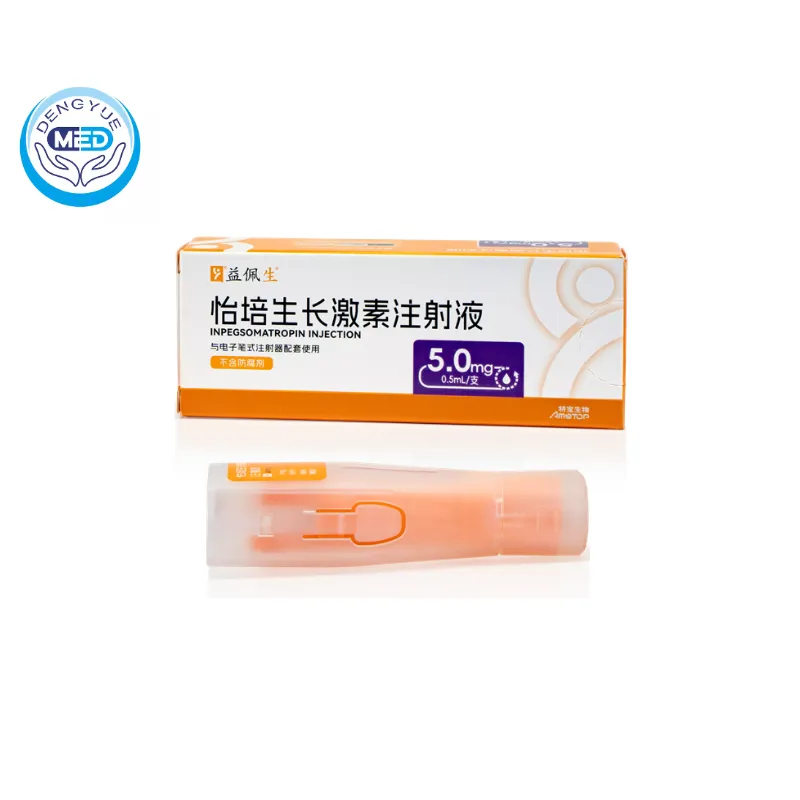
Inpegsomatropin Injection Characteristics
-
Ingredients: Active Ingredient: Inpegsomatropin (a long-acting pegylated recombinant human growth hormone).
-
Properties: Clear to slightly opalescent solution for injection. Long-acting formulation designed for once-weekly administration.
-
Packaging Specification: Prefilled pen / cartridge [specify strength, e.g., 15 mg/1.5 mL].
-
Storage: Store in a refrigerator at 2–8 °C. Do not freeze. Protect from light.
-
Expiry Date: As indicated on the package.
-
Executive Standard: As indicated on the package.
-
Approval Number: As indicated on the package.
-
Date of Revision: As indicated on the package.
-
Manufacturer: As indicated on the package.
Guidelines for the Use of Inpegsomatropin Injection
-
Dosage and Administration:
-
Administer subcutaneously once weekly.
-
Dose should be individualized based on body weight, clinical response, and IGF-1 levels.
-
Recommended Dose: As indicated on the package.
-
Administration:
-
Inject into the abdomen, thigh, or upper arm.
-
Rotate injection sites to avoid lipoatrophy.
-
-
Missed Dose:
-
If a dose is missed, administer as soon as possible within 3 days.
-
If more than 3 days have passed, skip the missed dose and resume the regular schedule.
-
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
Injection site reactions (pain, redness, swelling).
-
Headache.
-
Edema.
-
Arthralgia or muscle pain.
-
-
Serious Adverse Reactions:
-
Intracranial hypertension.
-
Slipped capital femoral epiphysis.
-
Increased risk of neoplasm recurrence.
-
Severe allergic reactions.
-
-
-
Contraindications:
-
Active malignancy.
-
Acute critical illness (post-surgery, trauma, respiratory failure).
-
Known hypersensitivity to Inpegsomatropin or excipients.
-
-
Precautions:
-
Monitor IGF-1 levels during therapy.
-
Caution in patients with diabetes mellitus or impaired glucose tolerance.
-
Adjust dose in patients with renal or hepatic impairment.
-
Monitor for signs of intracranial hypertension.
-
Inpegsomatropin Injection Interactions
-
Concomitant glucocorticoid therapy may reduce efficacy.
-
May affect metabolism of insulin and oral hypoglycemic agents (dose adjustment may be required).
-
Interaction with drugs metabolized by CYP450 enzymes is possible.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.









Reviews
There are no reviews yet.