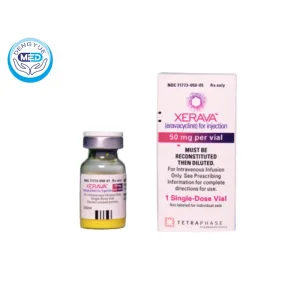
Xerava (Eravacycline) | CIAIs | HongKong DengYue Medicine
- Generic Name/Brand Name: Eravacycline/XERAVA
- Indications: CIAIs (Antibiotics)
- Dosage Form: Injection, lyophilized powder for solution
- Specification: 50mg x 1 vial
Eravacycline Application Scope
Intravenous treatment of complicated intra-abdominal infections (cIAIs) in adults caused by susceptible organisms (e.g., E. coli, K. pneumoniae, Enterococcus spp., S. aureus, Bacteroides spp., etc.)
Characteristics
-
Ingredients: 50 mg eravacycline (equivalent to 63.5 mg eravacycline dihydrochloride) per single-dose vial (lyophilized powder)
-
Properties: Fully synthetic fluorocycline (tetracycline class), with broad-spectrum activity including many MDR Gram-negative, Gram-positive, and anaerobic bacteria
-
Packaging Specification: Single-dose lyophilized powder vial containing 50 mg eravacycline
-
Storage: See prescribing info for optimal storage; generally stored at controlled room temperature before reconstitution
-
Expiry Date: Per vial label (usually 2–3 years from manufacture; check package insert)
-
Executive Standard: Complies with FDA/EMA prescribing standards as defined in XERAVA’s label
-
Approval Number: FDA-approved on August 27, 2018 for cIAI
-
Date of Revision: See latest FDA label (2018; check your package information for updated revision dates)
-
Manufacturer: Marketed by Innoviva Specialty Therapeutics on behalf of Tetraphase Pharmaceuticals
Guidelines for the Use of Eravacycline
-
Dosage and Administration:
-
Reconstitute vial and administer as IV infusion over ~60 minutes, every 12 hours
-
Recommended dose: 1 mg/kg IV every 12 hours for 4–14 days, based on infection severity and clinical response
-
Compatible with 0.9% sodium chloride; do not mix with other drugs
-
-
Adverse Reactions:
-
Common (≥3%): Infusion site reactions (≈7.7%), nausea (≈6.5%), vomiting (≈3.7%)
-
Others: Hives, difficulty breathing, diarrhea (including up to 2 months post-treatment), severe headaches, pancreatitis, photosensitivity, pseudotumor cerebri, elevated liver enzymes, BUN, and azotemia
-
-
Contraindications: Hypersensitivity to eravacycline, tetracycline-class antibiotics, or any excipient
-
Precautions:
-
Allergic reactions: Risk of anaphylaxis—discontinue use if symptoms occur
-
Tooth discoloration & enamel hypoplasia: Avoid during tooth development (pregnancy 2nd–3rd trimester, infants, children ≤8 years)
-
Bone growth inhibition: Possible reversible effects on bone growth in young children/pregnant women
-
Clostridioides difficile–associated diarrhea: Monitor for diarrhea during and up to 2 months post-therapy
-
Photosensitivity, pseudotumor cerebri, liver/kidney function changes, pancreatitis: Monitor and discontinue if suspected
-
Superinfection risk: May lead to fungal or resistant bacterial overgrowth; monitor and treat accordingly
-
Interactions
- Drug Interactions:
-
CYP3A inducers: e.g., rifampin—may reduce eravacycline exposure; dose adjustments may be needed
-
Anticoagulants: Additive effect—monitor INR and adjust dose as needed
-
General tetracycline-like interactions: Avoid coadministration with antacids, iron, calcium-rich foods that inhibit absorption (if/when oral formulation used)
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.

Reviews
There are no reviews yet.