Anastrozole Tablets | Early Breast Cancer | HK DengYueMed
- Generic Name/Brand Name: Anastrozole Tablets/Rittin
- Indications: Treatment of hormone receptor-positive breast cancer, prevention of recurrence, and chemoprevention in high-risk postmenopausal women.
- Dosage Form: Film-coated tablet
- Specification: 1mg
Anastrozole Tablets Application Scope
Anastrozole Tablets are indicated for the treatment of advanced breast cancer in postmenopausal women. Anastrozole Tablets may be considered in estrogen receptor-negative patients who show a positive clinical response to tamoxifen.
For the adjuvant treatment of hormone receptor-positive early breast cancer in postmenopausal women. Indicated for the adjuvant treatment of hormone receptor-positive early breast cancer in postmenopausal women who have received 2 to 3 years of adjuvant tamoxifen therapy.
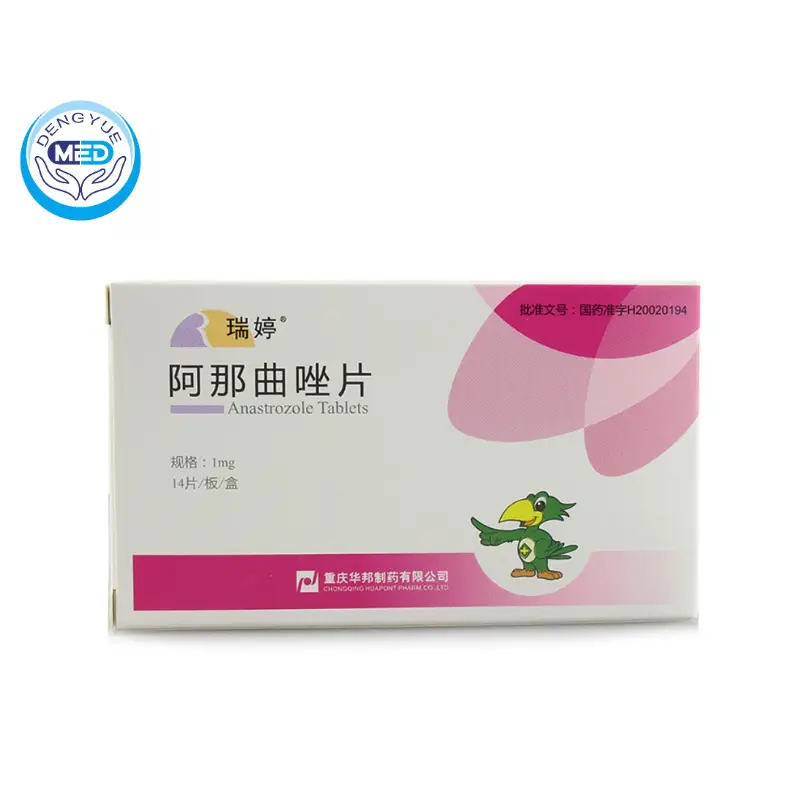
Anastrozole Tablets Characteristics
Ingredients: Anastrozole.
Characteristics: Treatment of breast cancer
Specification: 1mg
Packing: 14*1mg
Storage: Shielded from light, sealed.
Effective period: 18 months
Approval number: State Drug License H20020194
Date of Revision: According to the latest drug instructions.
Manufacturer: Chongqing Huabang Pharmaceutical Co.
Guidelines For The Use Of Anastrozole Tablets
Dosage and Administration: Take 1 tablet (1 mg) orally once daily. No dosage adjustment is usually necessary in patients with mild hepatic or mild to moderate renal impairment.
Adverse Reactions: These include flushing of the skin, vaginal dryness, overproduction of hair oil, gastrointestinal disturbances (anorexia, nausea, vomiting, and diarrhea), fatigue, depression, headache, or rash.
Anastrozole Tablets Medication Limitations
Contraindications: Premenopausal women, pregnant or lactating women.
Precautions: There is no information on the safety of Anastrozole Tablets in patients with moderate to severe hepatic impairment and severe renal impairment. When hormone levels are in doubt, amenorrhea should be considered to be the result of disruption of hormonal balance. The product has no significant effect on the patient’s ability to drive or operate machinery. However, symptoms of fatigue and depression have been reported in some patients, and special care should be taken when driving and operating machinery when these symptoms persist.
Anastrozole Tablets Interactions
Clinical interaction studies with antipyrine and metformin have shown that this product is less likely to cause cytochrome P-450-mediated drug reactions when used in combination with other drugs.
Clinical trials have not found significant interactions between this product and other commonly used clinical drugs. There is no evidence to indicate whether this product should be used in combination with other antitumor agents.
Estrogen-containing therapies may reduce the efficacy of this product and should not be used in combination with this product.
Do not use this product in combination with estrogen-containing therapies. Before starting treatment, tell your doctor about all medications you are using.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from HongKong DengYue Medicine, China Drug Import and Export Wholesaler.
It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.






Reviews
There are no reviews yet.