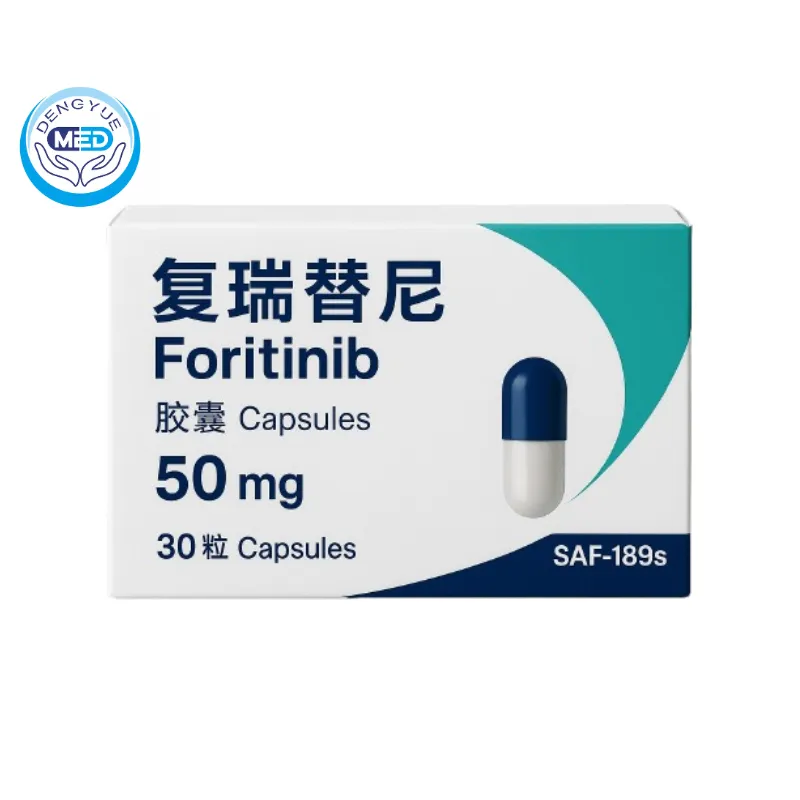
Fosun (Foritinib) – NSCLC | HongKong DengYue Medicine
- Generic Name/Brand Name: Foritinib/Fosun
- Indications: NSCLC
- Dosage Form: Capsules
- Specification: 50 mg × 30
Foritinib Application Scope
Foritinib (SAF-189s, foritinib succinate) is an investigational drug primarily intended for the treatment of anaplastic lymphoma kinase (ALK)-positive locally advanced or metastatic non-small cell lung cancer (NSCLC). It is also being evaluated for ROS1-positive NSCLC. As of December 2025, the drug’s marketing authorization application was accepted by China’s NMPA in March 2025 but voluntarily withdrawn in October 2025 to refine registration materials, with plans for resubmission. It has not yet received formal approval for marketing.
Foritinib Characteristics
-
Ingredients: Foritinib succinate, an innovative small-molecule chemical compound.
-
Properties: Capsule formulation; it acts as a highly efficient ALK and ROS1 inhibitor with strong central nervous system (CNS) penetration, enabling effectiveness against brain metastases.
-
Packaging Specification: Not applicable (drug not yet marketed; specifications from clinical trials include 50mg capsules).
-
Storage: Not specified in available data (as the drug is investigational; standard storage for similar capsules would be at room temperature, protected from moisture and light, but consult trial protocols).
-
Expiry Date: Not applicable (investigational status).
-
Executive Standard: Not applicable (no formal approval yet).
-
Approval Number: None (marketing application withdrawn; no NMPA approval as of December 2025).
-
Date of Revision: Not applicable.
-
Manufacturer: Fosun Wanbang (a subsidiary of Fosun Pharma), in collaboration with Shanghai Institute of Materia Medica, Chinese Academy of Sciences, and Chongqing Fochon Pharmaceutical Research.
Guidelines for the Use of Foritinib
-
Dosage and Administration:
-
Recommended Dose: 160mg orally once daily (based on Phase III clinical trial data). Earlier Phase I trials explored doses ranging from 20mg to 210mg daily.
-
Administration: Oral capsules, taken continuously until disease progression, unacceptable toxicity, or withdrawal. Can be taken with or without food (specific guidance not detailed in trials; follow physician advice).
-
Missed Dose: Specific instructions not available; generally, for similar drugs, take the missed dose as soon as remembered unless close to the next dose, then resume normal schedule. Do not double dose.
-
-
Adverse Reactions:
-
Common Adverse Reactions: Hyperglycemia, elevated liver enzymes, gastrointestinal issues (e.g., nausea, diarrhea), and electrocardiogram QT prolongation. Most are mild to moderate.
-
Serious Adverse Reactions: Grade 3-4 events include hyperglycemia (up to 12% of patients), QT prolongation, and potential CNS-related issues. No new safety signals identified in trials, but monitoring is required.
-
-
Contraindications: Hypersensitivity to foritinib succinate or its components. Specific contraindications not fully established due to investigational status; avoid in patients with uncontrolled hyperglycemia or severe cardiac conditions based on adverse event profiles.
-
Precautions:
- Monitor blood glucose levels regularly due to hyperglycemia risk. ECG monitoring for QT prolongation. Liver function tests recommended. Use caution in patients with CNS metastases (though the drug has good penetration). Dose adjustments may be needed for toxicities.
Foritinib Interactions
- Drug-drug interactions: Specific data limited, but as a tyrosine kinase inhibitor, potential interactions with strong CYP3A inhibitors (e.g., itraconazole) or inducers may affect exposure. A clinical trial (CTR20241820) evaluated interactions with itraconazole. Consult physician for concurrent medications.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.







Reviews
There are no reviews yet.