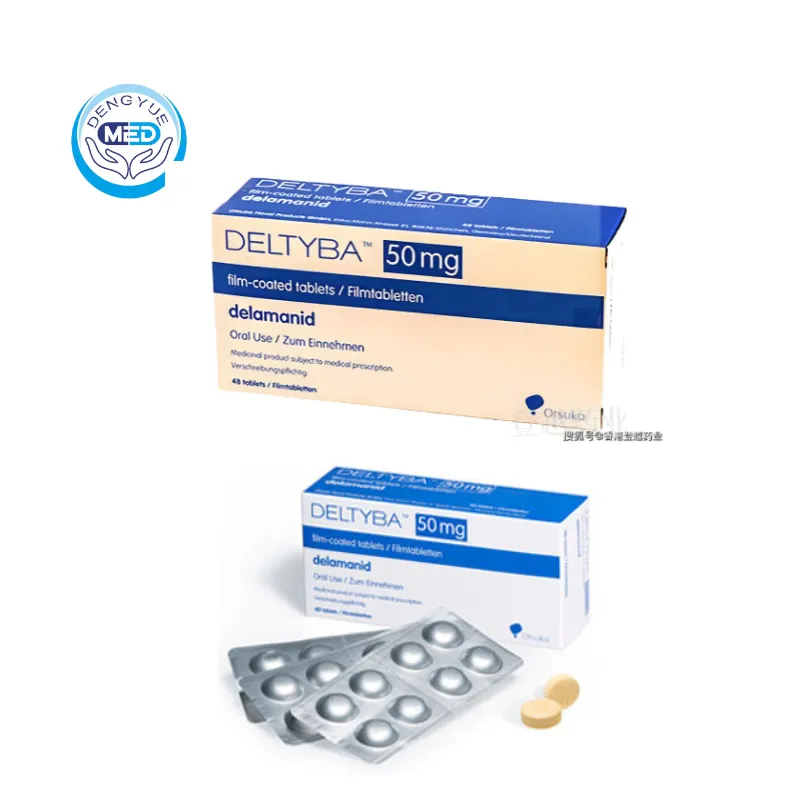
Deltyba (delamanid) – MDR-TB | HongKong DengYue Medicine
- Generic Name/Brand Name:Delamanid/Deltyba™
- Indications:MDR-TB
- Dosage Form: Film-coated tablet,Dispersible tablet
- Specification: 50 mg film-coated tablet/per pack
Delamanid Application Scope
Delamanid is indicated for the treatment of pulmonary multidrug-resistant tuberculosis (MDR-TB) as part of an appropriate combination regimen in:
-
Adults and adolescents (≥6 years old) with confirmed MDR-TB
-
Children aged ≥3 years (using age-appropriate formulations)
-
When effective treatment cannot otherwise be provided due to resistance or intolerance

deltyba delamanid
Delamanid Characteristics
-
Ingredients:
-
Active: Delamanid 50 mg per film-coated tablet (or 25 mg dispersible tablet in pediatrics)
-
Excipients: includes lactose monohydrate (100 mg per 50 mg tablet); full list in SmPC section 6.1
-
-
Properties:
-
Oral nitro‑imidazooxazole‑class antibiotic that inhibits the synthesis of mycolic acids in Mycobacterium tuberculosis, causing bacterial cell‑wall destabilization and death
-
Bactericidal agent active against MDR‑TB when used in combination regimen
-
-
Packaging Specification:Round, yellow film‑coated tablets, 11.7 mm diameter, debossed with “DLM” and “50” (for 50 mg tablet), or round white to off‑white dispersible tablets for pediatric strengths
-
Storage:Store at room temperature; protect from moisture; follow local label storage specifics (SmPC)
-
Expiry Date:As per packaging label; typically 24 months (check manufacturing label specifics). Standard expiry is end of stated month on the label. (Exact shelf‑life not specified in sources.)
-
Executive Standard: EMA EU marketing authorisation under EU/1/13/875 for Deltyba® for MDR‑TB therapy
-
Approval Number:
-
EMA conditional marketing authorisation granted April 28, 2014 (Deltyba®)
-
Also approved by Japan’s PMDA in July 2014 (as OPC‑67683 / Deltyba)
-
-
Date of Revision:Most recent SmPC revision published ~4 months ago per EMA product information document accessed recently
-
Manufacturer:Otsuka Pharmaceutical Co., Ltd. (Japan), marketed in EU and globally under the brand name Deltyba®
Guidelines for the Use of Deltyba
-
Dosage and Administration:
-
Recommended Dose: 100 mg twice daily (total daily dose 200 mg) taken orally for 24 weeks, in combination with an appropriate multidrug regimen for MDR-TB.
For pediatric patients (≥6 years and ≥20 kg): 50 mg twice daily (total 100 mg/day). -
Administration: Administer orally with food to improve absorption. Tablets should be swallowed whole with water and not crushed or chewed. Pediatric dispersible tablets may be dispersed in water for easier administration.
-
Missed Dose: If a dose is missed, it should be taken as soon as possible on the same day. If a full day is missed, do not double the dose the next day; continue with the regular dosing schedule.
-
-
Adverse Reactions:
-
Common Adverse Reactions (≥5% and >3% higher than placebo):
-
Nausea
-
Vomiting
-
Dizziness
-
QT prolongation
-
Hypokalemia
-
-
Serious Adverse Reactions:
-
QT prolongation with potential risk of cardiac arrhythmia
-
Hypokalemia requiring correction
-
Hepatic enzyme elevations (ALT, AST) in some patients
-
Electrocardiogram (ECG) abnormalities
-
-
-
Contraindications:
-
Known hypersensitivity to delamanid or any excipients
-
Severe liver impairment (Child-Pugh C)
-
QTc interval >500 ms at baseline
-
Concomitant use of strong CYP3A4 inducers (e.g., rifampicin, carbamazepine)
-
-
Precautions:
QT Prolongation:
Monitor ECG and electrolytes (K⁺, Mg²⁺, Ca²⁺) regularly. Avoid concomitant QT-prolonging drugs when possible.Hepatic Function:
Use with caution in patients with hepatic impairment; monitor liver enzymes regularly.Pregnancy and Lactation:
Not recommended during pregnancy or breastfeeding unless clearly needed. Women of childbearing potential should use effective contraception during treatment and for 5 months after.
Delamanid Interactions
- Delamanid may interact with drugs that prolong the QT interval or induce CYP3A4 enzymes, such as rifampicin, which can reduce its effectiveness or increase cardiac risk.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.





Reviews
There are no reviews yet.