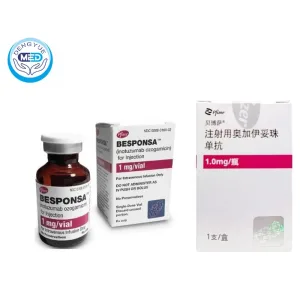
Besponsa (Inotuzumab Ozogamicin) – B-ALL | HongKong DengYue Medicine
- Generic Name/Brand Name: Inotuzumab Ozogamicin / Besponsa
- Indications: (R/R) CD22-positive B-cell precursor acute lymphoblastic leukemia (B-ALL)
- Dosage Form: Intravenous injection
- Specification: 1 mg × 1 vial
Besponsa Application Scope
Besponsa is indicated as monotherapy for the treatment of relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia (ALL) in adult patients.
For adult patients with Philadelphia chromosome–positive (Ph⁺) B-cell precursor ALL, Besponsa is indicated only in those who have failed treatment with at least one tyrosine kinase inhibitor (TKI).
In the U.S., it has also been approved for pediatric patients aged ≥ 1 year with relapsed or refractory CD22-positive B-cell precursor ALL.
Besponsa Characteristics
-
Ingredients: Inotuzumab Ozogamicin
-
Properties: Sterile, white to off-white, preservative-free lyophilized
-
Packaging Specification: Each single-dose vial contains 1 mg equivalent of inotuzumab ozogamicin
-
Storage: Store lyophilized vials at 2–8°C, protected from light. Do not freeze
-
Expiry Date: As indicated on the vial; reconstituted solution must be used within recommended timeframe
-
Executive Standard: Complies with regulatory standards (e.g., U.S. Pharmacopeia, EU/EP, or local pharmacopeia depending on region)
-
Approval Number: Varies by country/region; refer to local regulatory authority (e.g., FDA, EMA, NMPA)
-
Date of Revision: The last revision date of the prescribing information is indicated in the official documentation
-
Manufacturer: Pfizer/Wyeth
Guidelines for the Use of Besponsa
-
Dosage and Administration:
-
Recommended Dose: Dosage is calculated based on body surface area (BSA) and is administered over a treatment cycle.
-
Cycle 1: Total dose of 1.8 mg/m², administered as 0.8 mg/m² on Day 1 and 0.5 mg/m² on Day 8 and Day 15. The cycle duration is 3 weeks but can be extended to 4 weeks.
-
Subsequent Cycles: Dose varies based on treatment response (whether Complete Remission/CR with incomplete hematologic recovery/CRi is achieved)
-
-
Administration:
-
Pre-medication: Administer a corticosteroid, antipyretic, and antihistamine prior to each dose to reduce the risk of infusion-related reactions.
-
Infusion: The reconstituted and diluted solution is administered as an intravenous infusion over 1 hour. It must not be administered as an intravenous push or bolus.
-
-
Missed Dose:
-
If a scheduled dose is missed, it may be given as soon as possible, provided at least 6 days have passed since the preceding dose (to maintain minimum interval).
-
If interruption is prolonged (e.g., ≥14 days), dose reductions or omission of doses may be needed, or permanent discontinuation considered per safety guidance.
-
-
-
Adverse Reactions:
-
Common Adverse Reactions:
Some of the frequently observed adverse reactions include:
-
Hematologic toxicities: thrombocytopenia, neutropenia, anemia, leukopenia
-
Infections (including febrile neutropenia)
-
Elevated liver enzymes, hyperbilirubinemia, transaminase increase
-
Hemorrhagic events (bleeding)
-
Fatigue, fever, nausea, abdominal pain, headache
-
Infusion-related reactions (e.g. chills, hypotension, rash)
-
-
Serious Adverse Reactions:
-
Hepatic veno-occlusive disease (VOD) (also called sinusoidal obstruction syndrome)—boxed warning. Risk is especially elevated in patients who later undergo HSCT. Some cases have been fatal.
-
Severe liver toxicity (e.g., bilirubin, AST/ALT elevation) requiring discontinuation.
-
Severe infusion reactions (which may require interruption, treatment, or discontinuation).
-
Fatal outcomes in some cases have occurred, especially in the context of transplant or in patients with impaired hepatic reserve.
-
-
-
Contraindications:
-
Known hypersensitivity to inotuzumab ozogamicin or any component of the formulation.
-
Severe hepatic impairment (as per label)—patients with significant liver dysfunction are at elevated risk for hepatic toxicity/VOD.
-
-
Precautions:
-
Hepatic VOD: Monitor liver tests before and during treatment. Risk factors include pre-existing liver disease, prior HSCT, and older age.
-
Myelosuppression: Monitor complete blood counts regularly for signs of infection, bleeding, or other effects.
-
Infusion-Related Reactions: Monitor during and for at least 1 hour after infusion. Interrupt infusion and manage symptoms if a reaction occurs.
-
QTc Prolongation: Monitor electrolytes and ECGs in patients with risk factors or those taking concomitant drugs that prolong QT interval.
-
Embryo-Fetal Toxicity: Can cause fetal harm. Advise effective contraception during treatment and for several months after the last dose for both females and males. Avoid during pregnancy and breastfeeding.
-
Besponsa Interactions
- QT-Prolonging Drugs: Concomitant use with drugs known to prolong QT interval may increase the risk of cardiac arrhythmias. Avoid use or consider alternatives.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.







Reviews
There are no reviews yet.