
China Approves Inpegsomatropin Injection for Children with Growth Hormone Deficiency
China’s National Medical Products Administration (NMPA) has officially approved Inpegsomatropin Injection, a first-in-class, innovative long-acting growth hormone developed by Xiamen Amoytop Biotech Co., Ltd., for the treatment of growth hormone deficiency (GHD) in children aged 3 years and older.
👉 This approval marks a major milestone in providing safer, more effective, and convenient treatment options for children affected by GHD.
A Breakthrough in Pediatric GHD Treatment
Growth hormone deficiency (GHD) is an endocrine disorder caused by insufficient secretion of endogenous growth hormone, characterized primarily by short stature.
😫 Untreated GHD in children can lead to long-term metabolic disorders, psychological challenges, and increased risk of chronic diseases, significantly affecting life quality and overall health.

👉 Early diagnosis and timely growth hormone replacement therapy are essential for improving height, optimizing metabolic outcomes, and supporting healthy development into adulthood.
😔 Traditionally, recombinant human growth hormone (rhGH) requires daily injections, which often result in pain, inconvenience, and poor adherence.
Studies indicate that up to two-thirds of children miss at least one injection per week, directly impacting growth outcomes.
Innovative Long-Acting Technology
✨ Inpegsomatropin Injection is the world’s first Y-shaped 40kD PEGylated long-acting growth hormone, independently developed in China.
Its two Y-shaped PEG chains are linked to the hormone via highly stable amide and C–N bonds, significantly extending the half-life while maintaining high bioactivity.
✅ As the first long-acting growth hormone expressed in a eukaryotic system, its structure closely resembles endogenous growth hormone, enabling lower initial dosing and reduced immunogenicity.
🔬 Pharmacokinetic studies show that Inpegsomatropin injection has a half-life of approximately 65–120 hours, supporting once-weekly dosing, compared with daily injections required for short-acting rhGH.
Clinical Evidence Supporting Safety and Efficacy
A Phase III multicenter, open-label, randomized trial was conducted at 23 centers across China with 391 children diagnosed with GHD.
Participants were randomized 2:1 to receive either weekly Pegpesen (140 μg/kg/week) or daily rhGH (245 μg/kg/week) for 52 weeks.
The primary endpoint was annual growth velocity (GV, cm/year).
👉 Results demonstrated that at 52 weeks, the LS mean GV for the Pegpesen group was 9.910 cm/year, compared with 10.037 cm/year for the daily rhGH group, with an LS mean difference of –0.127, confirming that weekly Yipeisheng is non-inferior to daily rhGH.
Inpegsomatropin injection also offers significant safety and convenience benefits:
- 💉 Reduced injection frequency—only once weekly, reducing the annual injection burden by 313 injections.
- 🤒 Lower immunogenicity—clinical studies reported 0% incidence of new anti-drug or neutralizing antibodies.
- 💪 Improved adherence and quality of life—simplified “single-use prefilled” design, preservative-free formulation, and flexible dosing (0.14–0.28 mg/kg/week) help reduce risk and ease treatment.
To enhance convenience and adherence, Xiamen Amoytop Biotech has also developed a dedicated electronic auto-injector pen for Inpegsomatropin injection.
☀️ The device simplifies injection, provides voice-guided instructions, records dosing data in real-time, and integrates with a mobile app for personalized health management and physician data sharing.
Inpegsomatropin Injection—A Milestone for Pediatric Endocrinology in China
👉 Starting June 19, 2025, Inpegsomatropin injection has been introduced in over 300 hospitals across 27 provinces, marking the start of a new era in long-acting growth hormone therapy in China.
As a first-in-class biologic, Inpegsomatropin injection represents a significant advancement in the treatment and management of pediatric GHD, offering children and families a safer, more effective, and more convenient option.
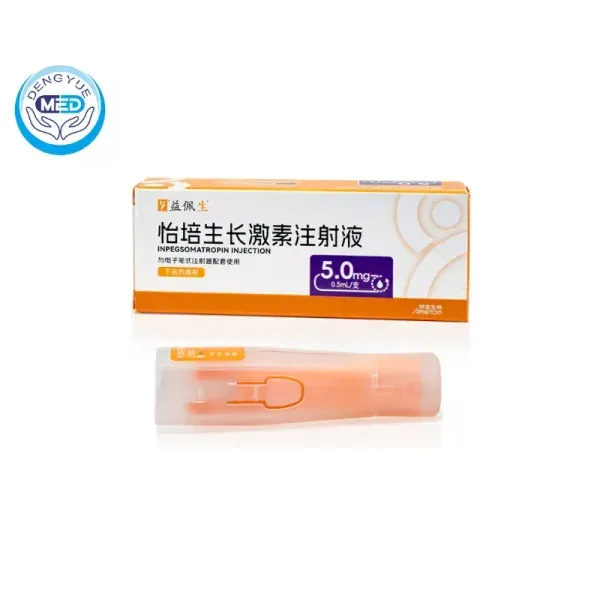
Pegpesen (Inpegsomatropin Injection) – AGHD | HongKong DengYue Medicine
- Generic Name/Brand Name: Inpegsomatropin Injection/Pegpesen
- Indications: AGHD
- Dosage Form: Injection
- Specification: 5 mg x 1 syringe
DengYue Medicine will continue to follow the development of innovative drugs in China, providing the public with more information on new medications and supporting the healthy growth of children.
👆 Please be careful to distinguish real from fake drugs when buying them. If you don’t yet know how to do that, take a look at this article.



