
Zeprumetostat (EZH2 Inhibitor) Approved by NMPA—Hengrui Pharma Advances R/R PTCL Treatment Innovation
The National Medical Products Administration (NMPA) has officially approved Zeprumetostat Tablets (brand name: Airuijing, development code: SHR2554), a Class 1 innovative drug independently developed by Jiangsu Hengrui Pharmaceuticals.
✨ The approval covers the treatment of adult patients with relapsed or refractory peripheral T-cell lymphoma (R/R PTCL) who have received at least one prior systemic therapy.
✨ As China’s first domestically developed EZH2 inhibitor, Zeprumetostat represents a major advancement in hematologic oncology and epigenetic drug innovation.
Innovative Mechanism: Targeting EZH2 to Reverse Epigenetic Dysregulation
💊 Zeprumetostat is a novel, potent, and selective oral EZH2 inhibitor capable of effectively suppressing both wild-type and mutant EZH2 enzyme activity.
🧬 EZH2, a histone methyltransferase responsible for H3K27 trimethylation (H3K27me3), plays a key role in gene silencing and tumorigenesis.
Its dysregulation is associated with multiple cancers, including peripheral T-cell lymphoma.
🔬 By inhibiting EZH2, Zeprumetostat disrupts aberrant histone methylation, induces G1 cell cycle arrest and apoptosis, and suppresses the growth of lymphoma and other malignancies both in vitro and in vivo.
This unique mechanism overcomes limitations of traditional chemotherapy and certain targeted therapies, providing new hope for patients with R/R PTCL.
Clinical Results: Durable Responses and Broad Efficacy
Data presented at the 2025 European Hematology Association (EHA) Annual Meeting demonstrated Zeprumetostat’s strong efficacy and manageable safety profile in patients with relapsed or refractory PTCL.
The pivotal, multi-center study led by Peking University Cancer Hospital enrolled 67 patients previously treated with chemotherapy and either chidamide or brentuximab vedotin.
Key results included:
- Complete response (CR): 32.8%
- Partial response (PR): 31.3%
- Overall response rate (ORR): 64.2% (assessed by independent review committee)
- Median duration of response (DoR): 18.7 months
- Median progression-free survival (PFS): 10.0 months
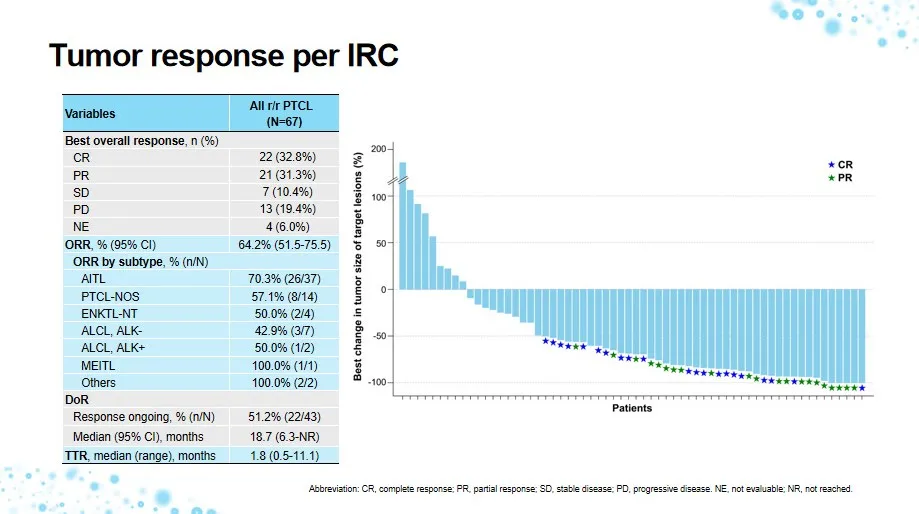
At data cutoff, 51.2% of responding patients remained in remission, underscoring the drug’s sustained anti-tumor activity across multiple PTCL subtypes.
Pipeline Expansion: From Lymphoma to Solid Tumors
Hengrui is currently conducting a Phase III head-to-head study (NCT06122389) comparing Zeprumetostat with chidamide in patients with T-cell lymphoma.
Beyond PTCL, Zeprumetostat is being investigated in several additional cancer types, including prostate cancer, colorectal cancer, and non-small cell lung cancer (NSCLC)—all in Phase II development—highlighting its broad potential in epigenetic-driven malignancies.
Global Collaboration: From China to the World
In 2023, Hengrui entered into a global licensing agreement with Treeline Biosciences, granting the company exclusive rights to develop, manufacture, and commercialize Zeprumetostat outside Greater China.
💰 Under the terms, Hengrui received a US$11 million upfront payment, up to US$45 million in development milestones, and up to US$650 million in potential sales milestones
This collaboration reflects global recognition of Chinese pharmaceutical innovation and marks an important step in Hengrui’s internationalization journey.
A New Milestone for China’s Innovative Oncology Pipeline
The approval of Zeprumetostat marks a breakthrough for domestically developed EZH2 inhibitors and underscores China’s growing capability in epigenetic and targeted drug discovery.
☀️ It offers a novel, effective therapeutic option for patients with R/R PTCL—one of the most challenging subtypes of lymphoma—and highlights the transformation of Chinese pharmaceutical innovation from “follower” to “leader.”
DengYueMed, as a professional pharmaceutical distributor focusing on innovative therapies, closely follows China’s progress in oncology innovation, including Hengrui Pharma’s development of Zeprumetostat.



