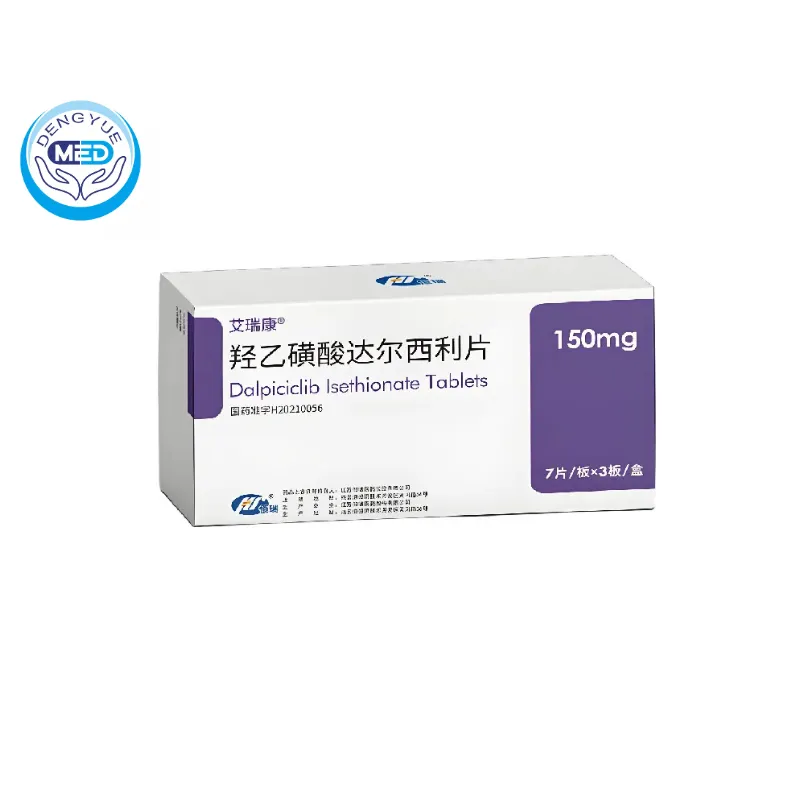
Airuikang (Dalpiciclib Isethionate) | Breast Cancer
- Generic Name/Brand Name: Dalpiciclib Isethionate/AiRuiKang
- Indications: Metastatic breast cancer
- Dosage Form: Oral tablets/capsules
- Specification: 150 mg, 21 tablets (7 tablets × 3 blisters) per box
Dalpiciclib Isethionate Application Scope
-
Approved in China (Dec 31, 2021) in combination with fulvestrant for treatment of hormone receptor–positive (HR⁺), HER2-negative recurrent or metastatic breast cancer after progression on endocrine therapy
-
Under clinical investigation in HR⁺/HER2⁻ early and advanced breast cancer, castration‑sensitive prostate cancer, HER2-positive breast cancer, malignant melanoma, ovarian, and other solid tumors

Dalpiciclib Isethionate Characteristics
-
Ingredients:
-
Active: Dalpiciclib (cyclin-dependent kinase 4/6 inhibitor), isethionate salt
-
Excipients: Details not disclosed in public sources.
-
-
Properties: Oral, selective CDK4/6 small-molecule inhibitor; halts cell-cycle progression at G₁-S checkpoint by reducing Rb phosphorylation, inhibiting tumor proliferation
-
Packaging Specification: Tablets or capsules. Strengths unspecified in public sources; commonly administered as daily oral doses (e.g., 100 mg) in trials
-
Storage:
-
As research compound: powder 3 years at −20 °C; stock solutions: −80 °C for 6 months or −20 °C for 1 month
-
Commercial tablet storage likely per label (typically room temperature)—not publicly specified.
-
-
Expiry Date: Unknown
-
Executive Standard: Unknown
-
Approval Number: NMPA-approved for HR⁺/HER2⁻ metastatic breast cancer on December 31 2021; exact registration number not public
-
Date of Revision: Unknown
-
Manufacturer: Jiangsu Hengrui Pharmaceuticals Co., Ltd.
Guidelines for the Use of Dalpiciclib Isethionate
-
Dosage and Administration:
-
Oral, once daily; in Phase I/III combination with endocrine agents like fulvestrant or letrozole, common dose ~100 mg/day .
-
Taken with or without food; duration dependent on response and tolerance; regular monitoring recommended.
-
-
Adverse Reactions:
-
Common: neutropenia, anemia, thrombocytopenia, fatigue, nausea, diarrhea, liver enzyme elevations
-
Less common: hepatotoxicity (requires liver monitoring), occasional QT prolongation, alopecia, rash
-
-
Contraindications: Severe hepatic impairment, pregnancy, breastfeeding; known hypersensitivity; caution in patients with severe bone marrow suppression
-
Precautions:
- Monitor CBCs, liver function, cardiac symptoms (ECG); adjust dose or interrupt therapy for severe cytopenias or liver toxicity
Interactions
- Drug Interactions:
-
Metabolized via CYP3A4: avoid strong inhibitors (e.g., ketoconazole) or inducers (e.g., rifampin), which can alter drug exposure
-
May have additive myelosuppressive or hepatotoxic effects with other chemotherapy or targeted agents.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.







Reviews
There are no reviews yet.