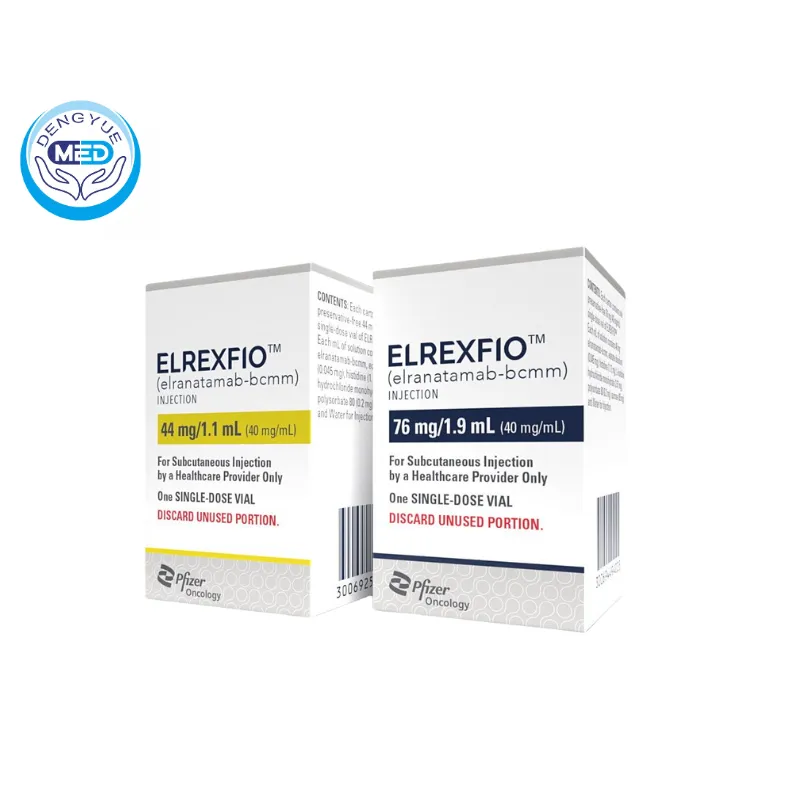
Elranatamab|Multiple Myeloma|HongKong DengYue Medicine
- Generic Name/Brand Name: Elranatamab / Elrexfio
- Indications: Multiple Myeloma
- Dosage Form: Subcutaneous injection
- Specification: 76 mg/1.9 mL (40 mg/mL)
Elranatamab Application Scope
-
Indications: Elranatamab is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
Elranatamab Characteristics
-
Ingredients: Elranatamab-bcmm (active ingredient)
-
Properties: Elranatamab is a bispecific B-cell maturation antigen (BCMA)-directed CD3 T-cell engager. It is a humanized bispecific antibody that binds to BCMA on multiple myeloma cells and CD3 on T-cells, facilitating T-cell-mediated cytotoxicity against BCMA-expressing cells.
-
Specification: Injection: 76 mg/1.9 mL (40 mg/mL) in a single-dose vial.
-
Packaging Specification: Single-dose vial containing 76 mg/1.9 mL solution.
-
Storage: Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze.
-
Expiry Date: Refer to the expiration date printed on the vial and carton.
-
Executive Standard: Approved under the U.S. FDA’s accelerated approval program pursuant to section 506(c) of the Federal Food, Drug, and Cosmetic Act (FDCA) and 21 CFR 601.41.
-
Approval Number: Biologics License Application (BLA) number 761345.
-
Date of Revision: August 2023.
-
Manufacturer: Pfizer Inc.
Guidelines for the Use of Elranatamab
-
Dosage and Administration: Elranatamab is administered subcutaneously with an initial step-up dosing schedule to mitigate the risk of cytokine release syndrome (CRS). The recommended dosing regimen is as follows:
-
Step-up dose 1: 12 mg on Day 1
-
Step-up dose 2: 32 mg on Day 4
-
First treatment dose: 76 mg on Day 8
-
Maintenance dosing: 76 mg weekly for the first 24 weeks, then every two weeks thereafter until disease progression or unacceptable toxicity.
-
-
Adverse Reactions: Common adverse reactions include:
-
Cytokine release syndrome (CRS)
-
Fatigue
-
Injection site reactions
-
Pyrexia (fever)
-
Diarrhea
-
Upper respiratory tract infections
-
Musculoskeletal pain
-
Decreased appetite
-
Cough
-
Nausea
-
Headache
-
Peripheral neuropathy
-
Anemia
-
Neutropenia
-
Thrombocytopenia
-
Increased liver enzymes
-
-
Contraindications: None listed.
-
Precautions:
-
Cytokine Release Syndrome (CRS): Monitor patients for signs and symptoms of CRS, including fever, hypotension, hypoxia, and organ dysfunction. Administer premedications and manage CRS according to severity.
-
Neurologic Toxicity: Monitor for signs of neurologic toxicity, including immune effector cell-associated neurotoxicity syndrome (ICANS).
-
Infections: Monitor for signs and symptoms of infection during and after treatment.
-
Hematologic Toxicities: Monitor complete blood counts during treatment.
-
Embryo-Fetal Toxicity: Elranatamab may cause fetal harm. Advise females of reproductive potential to use effective contraception during treatment and for a specified period after the last dose.
-
Elranatamab Interactions
-
Drug Interactions: Elranatamab can cause cytokine release syndrome (CRS), which may suppress the activity of cytochrome P450 (CYP) enzymes, particularly CYP3A4. This suppression can lead to increased exposure of drugs metabolized by these enzymes. Caution is advised when co-administering elranatamab with CYP3A4 substrates, especially those with a narrow therapeutic index.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.






Reviews
There are no reviews yet.