
What Is the Mechanism of Rezvilutamide—A New Drug for Prostate Cancer
Prostate cancer remains one of the most commonly diagnosed malignancies among men worldwide, and despite significant advances in diagnosis and treatment, disease progression and therapeutic resistance continue to pose major clinical challenges.
Ariane (rezvilutamide) as a novel androgen receptor inhibitor, has emerged as a promising therapeutic option for its targeted mechanism, favorable pharmacological profile, and potential clinical value.
In this evolving therapeutic environment, Dengyue Medicine—the global drug wholesale distributor, closely follows global advances in oncology and emerging innovative treatments.
👉 This article provides a comprehensive overview of Ariane, with a particular focus on a key question: what is the mechanism of rezvilutamide? By examining its biological targets, therapeutic rationale, and clinical significance, we aim to offer a clear and structured understanding of this new drug and its place in the evolving prostate cancer treatment paradigm.
Removing the Sense of Unfamiliarity—What Exactly Is Ariane (rezvilutamide)?
When encountering the term Ariane Rezvilutamide Tablets, a clear definition is essential. From a pharmacological perspective, Ariane is the brand name, while rezvilutamide is the generic chemical name.
Rezvilutamide is an orally administered, small-molecule targeted therapy independently developed by a Chinese pharmaceutical company, with prostate cancer as its core indication. In terms of drug classification, it belongs to the class of second-generation androgen receptor (AR) inhibitors.
To understand its therapeutic role, it is necessary to begin with the biological basis of prostate cancer.
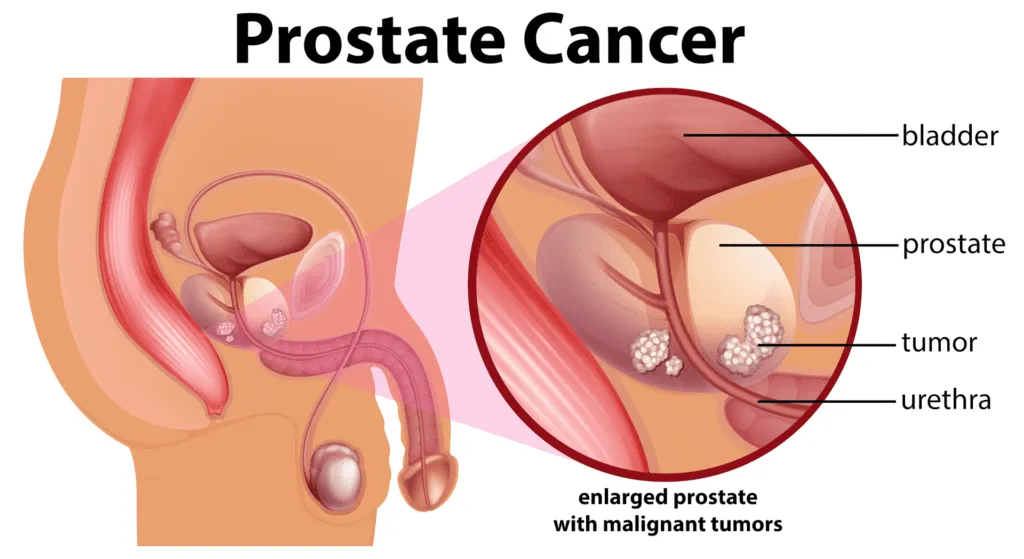
The proliferation and survival of prostate cancer cells are highly dependent on androgen signaling, primarily mediated by testosterone.
👉 This process is initiated when androgens bind to the androgen receptor within cancer cells. Upon ligand binding, the receptor undergoes conformational changes, translocates into the nucleus, and functions as a transcription factor, activating a cascade of gene expression programs that promote tumor cell growth, proliferation, and survival.
✨ The mechanism of action of rezvilutamide is characterized by precise and comprehensive interference with this critical signaling pathway.
.webp)
Rezvilutamide exhibits a high binding affinity for the androgen receptor and acts as a competitive antagonist, preventing endogenous androgens from engaging the receptor.
Importantly, its pharmacological effects extend beyond simple receptor blockade. Unlike first-generation AR inhibitors, rezvilutamide effectively suppresses androgen receptor nuclear translocation after receptor binding.
Even when limited receptor complexes enter the nucleus, rezvilutamide strongly inhibits their interaction with androgen response elements on DNA, thereby blocking downstream oncogenic gene transcription.
✨ Moreover, rezvilutamide promotes androgen receptor degradation through the ubiquitin–proteasome pathway, directly reducing intracellular levels of functional receptor protein.
Through this multi-pronged mechanism
- Competitive antagonism
- Inhibition of nuclear translocation
- Blockade of DNA binding
- Induction of receptor degradation
Rezvilutamide achieves a layered and durable suppression of androgen signaling. This results in potent inhibition of prostate cancer cell growth and the induction of tumor cell apoptosis.
Based on this distinctive and robust mechanism of action, rezvilutamide (Ariane) represents an advanced therapeutic option in the field of prostate cancer treatment. As a novel agent developed in China, it offers patients a clinically meaningful therapy supported by independent intellectual property and aligned with international standards.
Building Trust—Why Can Rezvilutamide Be Trusted?
In the field of medicine, the value and credibility of any innovative therapy must be grounded in rigorous, objective clinical evidence.
The clinical validation of rezvilutamide (Ariane) is derived from a pivotal Phase III, international, multicenter, randomized controlled trial known as the CHART study. The results of this well-designed trial were published in the authoritative journal The Lancet Oncology, providing the highest level of evidence-based support for the drug’s efficacy and safety.
Key Clinical Evidence: Survival Benefits Beyond Standard Therapy
The CHART study enrolled patients with high-risk metastatic hormone-sensitive prostate cancer (mHSPC). Its findings clearly demonstrated the significant advantages of rezvilutamide in combination with standard therapy compared with standard therapy alone.
- Breakthrough in Overall Survival (OS)
👉 The study showed that treatment with rezvilutamide resulted in a 56% reduction in the risk of death.
Overall survival is widely regarded as the gold-standard endpoint for evaluating anticancer therapies, as it directly reflects a meaningful extension of patients’ lives.
This benefit was not only statistically significant but also highly clinically relevant.
- Significant Improvement in Radiographic Progression-Free Survival (rPFS)
👉 In terms of disease control, rezvilutamide reduced the risk of radiographic disease progression or death by 54%. Median rPFS was not reached in the rezvilutamide group, compared with 25.1 months in the control group.
These results confirm that rezvilutamide can effectively prolong the period during which the disease remains controlled, delaying progression to the more challenging and treatment-limited castration-resistant stage, thereby helping patients maintain quality of life for a longer duration.
- Consistent Benefits Across Key Secondary Endpoints
👉 In addition to the primary endpoints, consistent advantages were observed across multiple secondary endpoints, including time to prostate-specific antigen (PSA) progression and time to subsequent skeletal-related events.
These findings further validate the breadth and durability of the therapeutic benefit provided by rezvilutamide.
Positioning Within the Therapeutic Landscape: A Multidimensional Perspective on Mechanism, Efficacy, and Safety
To fully appreciate the clinical value of rezvilutamide, it is important to consider its position within the current treatment paradigm for prostate cancer.
- Compared with First-Generation Androgen Receptor Inhibitors (e.g., bicalutamide)
First-generation agents are limited by incomplete receptor antagonism and, under certain conditions, may even exhibit partial agonist activity.
As a second-generation androgen receptor inhibitor, rezvilutamide demonstrates substantially higher receptor affinity and more complete antagonism, achieving durable and irreversible blockade at the pharmacological level and thereby avoiding the risks of insufficient efficacy or paradoxical tumor stimulation.
Bicalutamide Tablets | Advanced Prostate Cancer
- Generic Name/Brand Name: Bicalutamide/ZhaoHuiXian
- Indications: Luteinizing Hormone-releasing Hormone (LHRH), Advanced Prostate Cancer
- Dosage Form: Intravenous injection
- Specification: 50 mg
- Compared with Other Second-Generation Imported Agents (e.g., enzalutamide, apalutamide)
💡 The survival benefits observed in the CHART study are comparable to those reported in pivotal trials of leading global therapies, confirming that rezvilutamide meets international standards of efficacy on key clinical endpoints.
While delivering robust efficacy, rezvilutamide has demonstrated a predictable and manageable safety profile.
Common adverse events—such as elevations in blood lipids, liver enzymes, and fatigue—are generally consistent with those seen with other agents in this class, for which clinicians have well-established monitoring and management strategies.
Notably, CHART data indicate a relatively lower incidence of central nervous system–related adverse events, including seizures and severe fatigue, which may offer improved tolerability for certain patient populations.

Xtandi (Enzalutamide Soft Capsules) – Prostate Cancer | HongKong DengYue Medicine
- Generic Name/Brand Name: Enzalutamide / XTANDI®
- Indications: Metastatic castration-resistant prostate cancer (mCRPC), nmCRPC, mCSPC and certain high-risk hormone-sensitive prostate cancers.
- Dosage Form: Soft gelatin capsule
- Specification: 40 mg x 112 soft capsules
Rezvilutamide is neither a simple imitation nor a late follower. Through original research and rigorous clinical validation, it provides a new, evidence-based therapeutic option for patients in China and worldwide.
In real-world clinical practice, physicians can make more individualized treatment decisions among multiple effective second-generation agents, taking into account patient-specific factors such as comorbidities, concomitant medications, and concerns regarding particular adverse effects.
Practical Guidance—What Must You Know Before Using Rezvilutamide?
After understanding the value of the drug and the clinical evidence supporting it, the most critical question becomes practical: if a patient is eligible, how should rezvilutamide be used correctly and safely?
The following guidance is based on its approved indication and clinical practice principles.
Precise Positioning: Who should use It?
Rezvilutamide is not indicated for all patients with prostate cancer.
Its clinical use is clearly defined. Currently, in China, rezvilutamide is approved for the treatment of high-risk metastatic hormone-sensitive prostate cancer (mHSPC).
To clarify this term:
- Metastatic (M1): This indicates that the cancer has spread beyond the prostate gland.
- Imaging studies—such as bone scans, CT, MRI, or PSMA PET-CT—have confirmed metastatic lesions in the bones, distant lymph nodes, or other organs. This represents a critical stage in disease progression.
- Hormone-Sensitive Prostate Cancer (HSPC): This refers to a disease state in which tumor growth remains primarily driven by androgen (testosterone) signaling.
- After initiation of androgen deprivation therapy (ADT), tumor markers such as prostate-specific antigen (PSA) typically decline significantly, indicating disease control.
- This phase represents a key therapeutic window during which treatment intensification can yield long-term benefits.
- High Risk: Among patients with metastatic hormone-sensitive prostate cancer, risk stratification is performed based on factors such as the number and location of metastatic lesions, Gleason score, PSA levels, and other clinical parameters.
- “High-risk” disease generally reflects a more aggressive tumor biology and a greater need for intensified therapy to prevent rapid progression. The CHART trial specifically demonstrated the substantial benefit of rezvilutamide in this patient population.
In practical terms, if you have been diagnosed with prostate cancer, have confirmed metastatic disease, show a favorable response to initial hormone therapy, and are assessed by your physician as having high-risk features, you may be an appropriate candidate for rezvilutamide.
Standardized Use: Correct Administration and Therapeutic Foundations
Once eligibility is confirmed, standardized use is essential to ensure both efficacy and safety.
Rezvilutamide must be used in combination with androgen deprivation therapy (ADT). ADT typically involves medical castration using LHRH agonists or antagonists, or surgical castration.Rezvilutamide provides a deeper blockade of the androgen receptor signaling pathway on top of ADT.
The two approaches are synergistic and together achieve superior outcomes. Rezvilutamide should not be used as monotherapy.
- Dose: The recommended dose is 240 mg once daily (one tablet per day).
- Administration: Taken orally at a fixed time each day, with or without food, to promote adherence.
❗ Patients should not reduce the dose, interrupt, or discontinue treatment on their own, even if they feel well or are concerned about adverse effects. Any dose adjustments must be made by the physician based on individual clinical circumstances.
Ariane (Rezvilutamide) – mHSPC | HongKong DengYue Medicine
- Generic Name/Brand Name: Rezvilutamide/Ariane
- Indications: Prostate Cancer
- Dosage Form: Tablet
- Specification: 80 mg × 84 tablets
Proactive Management: Monitoring and Managing Adverse Effects
All effective cancer therapies may be associated with adverse effects. Early awareness and proactive management are essential components of successful treatment. Overall, the safety profile of rezvilutamide is well characterized and manageable.
- Common and Generally Manageable Adverse Effects
- Metabolic effects: Elevations in triglycerides and cholesterol; dietary modification and lipid-lowering therapy may be recommended when necessary.
- Hepatic effects: Increases in liver enzymes (ALT/AST); regular liver function monitoring is essential.
- Systemic effects: Fatigue and asthenia are common; appropriate balance between rest and activity is important.
- Others: Rash, pruritus, and weight gain may occur.
- Adverse Effects Requiring Special Attention
- Cardiac electrophysiological effects: Rezvilutamide may prolong the QT interval, a measure of cardiac repolarization on electrocardiography, which theoretically increases the risk of arrhythmias. Baseline and periodic ECG monitoring is therefore standard practice. Patients with pre-existing heart disease or those taking medications that may affect the QT interval should inform their physician.
- Central nervous system effects and fall risk: Some patients may experience dizziness, vertigo, or sensory disturbances, increasing the risk of falls, particularly in older individuals. Caution is advised when standing up, turning, or using stairs, and a safe home environment should be ensured.
In summary, the clinical use of rezvilutamide is a systematic process of precise patient selection, standardized administration, and proactive monitoring.
For appropriately selected patients, it represents a powerful therapeutic option. Ensuring its safe and effective use requires close collaboration, shared information, and mutual trust between patients and their healthcare providers.
Conclusion
💡 The introduction of rezvilutamide (Ariane) represents a major advance in the treatment of high-risk metastatic hormone-sensitive prostate cancer (mHSPC) in China. It not only addresses a critical unmet need in domestically developed innovative therapies, but also secures its clinical relevance through strong evidence-based validation.
Supported by national reimbursement, rezvilutamide has significantly improved access to advanced treatment and helped shift clinical practice toward earlier and more precise therapeutic intensification. Beyond its clinical impact, the drug reflects the growing capability of China’s pharmaceutical innovation ecosystem to deliver original, globally competitive oncology therapies.
As a Chinese innovative pharmaceutical import and export company, we closely follow such breakthroughs and remains committed to supporting the responsible dissemination of evidence-based therapies that improve patient outcomes worldwide.
FAQ about What Is the Mechanism of Rezvilutamide
What is the mechanism of action of LHRH agonists?
Continuous treatment with LHRH agonists causes a downregulation (a decrease in the number) of LHRH receptors and an uncoupling of the LHRH signal transduction mechanism.
What cancers can LHRH agonists treat?
Chronic administration of LHRH agonists is being used to induce the regression of endocrine-dependent malignant neoplasms, especially prostate and breast cancer.
What is the gold treatment for prostate cancer?
Gold-silica nanoshell therapy is an emerging targeted treatment modality for prostate cancer.
What kills prostate cancer cells naturally?
Allium vegetables: This group includes garlic, onions, leeks, and chives, have been shown to help kill prostate cancer cells.



